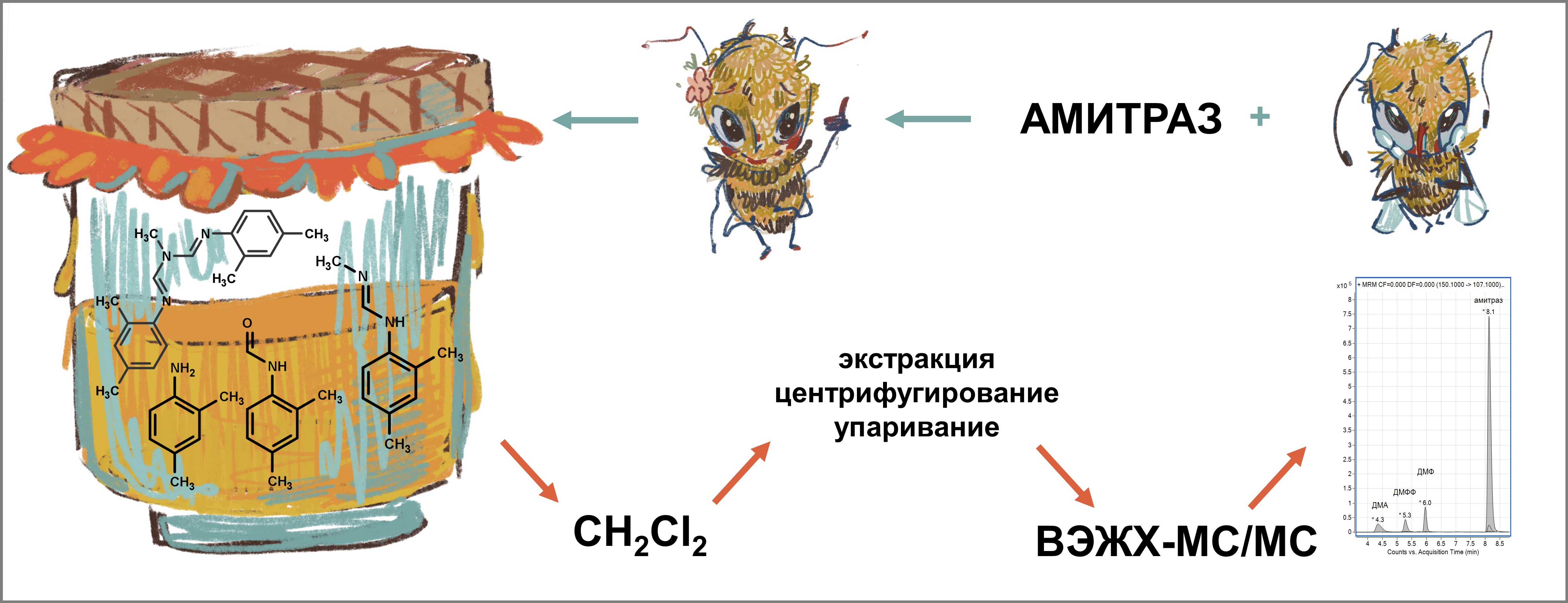
Разработка и валидация методики определения амитраза и его метаболитов в мёде методом ВЭЖХ–МС/МС
Аннотация
Разработали и валидировали селективную, простую в реализации, экспрессную методику совместного определения в мёде остаточного содержания инсектицида амитраз и трех его метаболитов (ДМФФ, ДМФ и ДМА). Методика основана на жидкость-жидкостной экстракции аналитов дихлорметаном из водного раствора мёда, подщелоченного аммиаком для улучшения стабильности лабильного в кислой среде инсектицида в процессе пробоподготовки. Получаемые экстракты достаточно чистые и не требуют дополнительной очистки с использованием твердофазной экстракции либо ее вариантов. Выбор экстрагента основан на предварительном изучении распределения амитраза, ДМФФ, ДМФ и ДМА в экстракционных системах вода – органический растворитель (н-гексан, толуол, дихлорметан, хлороформ). Количественное определение осуществляется методом ВЭЖХ–МС/МС в режиме мониторинга множественных реакций с использованием матричной градуировки в диапазоне массовой доли аналитов в мёде от 25 до 250 мкг/кг. Значения предела обнаружения в зависимости от аналита составляют от 0.2 до 2.3 мкг/кг, предела определения – от 0.6 до 4.6 мкг/кг, относительного стандартного отклонения повторяемости – от 1.4 до 5.8 %, относительного стандартного отклонения промежуточной прецизионности – от 1.4 до 7.7 %, относительной расширенной неопределенности – от 9 до 22 %. Градуировочные графики линейны в диапазоне массовой доли аналитов в мёде от 25 до 250 мкг/кг. Также в процессе валидации оценили инструментальные пределы обнаружения и определения, матричный эффект, степень извлечения, общую эффективность процесса, смещение.
Ключевые слова: амитраз, метаболиты, жидкость-жидкостная экстракция, ВЭЖХ–МС/МС, мёд.
Полный текст:
PDFЛитература
REFERENCES
Filazi A., Yurdakok-Dikmen B. Chapter 41 - Amitraz. Veterinary Toxicology 3rd ed. Basic and Clinical Principles ed. by R. C. Gupta. Academic Press, 2018. pp. 525-531. doi: 10.1016/B978-0-12-811410-0.00041-6.
Weijia Zheng, Jin-A. Park, A. M. Abd El-Aty, Seong-Kwan Kim, Sang-Hyun Cho, Jeong-min Choi, Hee Yi, Soo-Min Cho, Amer Ramadan, Ji Hoon Jeong, Jae-Han Shim, Ho-Chul Shin. Development and validation of modified QuEChERS method coupled with LC–MS/MS for simultaneous determination of cymiazole, fipronil, coumaphos, fluvalinate, amitraz, and its metabolite in various types of honey and royal jelly. J. Chromatogr. B, 2018, vol. 1072, pp. 60-69. doi: 10.1016/j.jchromb.2017.11.011.
Jin-Zhong Xu, Jian-Jun Miao, Hong Lin, Tao Ding, Zhen-Yun Zhao, Bin Wu, Chong-Yu Shen, Yuan Jiang. Determination of amitraz and 2,4-dimethylaniline residues in honey by using LC with UV detection and MS/MS. J. Sep. Sci., 2009, vol. 32, no. 23-24, pp. 4020-4024. doi: 10.1002/jssc.200900437.
Osano O., Oladimeji A. A., Kraak M. H., Admiraal W. Teratogenic effects of amitraz, 2,4-dimethylaniline, and paraquat on developing frog (Xenopus) embryos. Arch. Environ. Con. Tox., 2002, vol. 43, pp. 42-49. doi: 10.1007/s00244-002-1132-4.
Nilgun Tokman, Carla Soler, Marinel la Farré, Yolanda Picó, Damià Barceló. Determination of amitraz and its transformation products in pears by ethyl acetate extraction and liquid chromatography–tandem mass spectrometry. J. Chromatogr. A, 2009, vol. 1216, no. 15, pp. 3138-3146. doi: 10.1016/j.chroma.2009.01.099.
Corta E., Bakkali A., Berrueta L. A., Gallo B., Vicente F. Kinetics and mechanism of amitraz hydrolysis in aqueous media by HPLC and GC-MS. Talanta, 1999, vol. 48, no. 1, pp. 189-199. doi: 10.1016/S0039-9140(98)00237-9.
Charmaine M. van Eeden, Wilna Liebenberg, Jan L. du Preez, Melgardt M. de Villiers. Solvent and surfactant enhanced solubilization, stabilization, and degradation of amitraz. J. Environ. Sci. Heal. B, 2004, vol. B39, no. 1, pp. 33–51. doi: 10.1081/PFC-120027437.
GN 06/21/2013 No. 52. [Indicators of safety and harmlessness for humans of food raw materials and food products: GN 06/21/2013 No. 52: approved. Ministry of Health Republic of Belarus 06/21/2013 No. 52.]. Sbornik normativnykh dokumentov po prodovol'stvennomu syr'iu i pishchevym produktam [Collection of normative documents on food raw materials and food products]. Minsk, 2014. 235 p. (in Russian).
Council of the Eurasian Economic Commission. TR TS [Customs Union Technical Regulation] 021/2011. On the Safety of Food Products. Minsk, Energopress Publ., 2021. 144 p. (in Russian).
Commission Regulation (EU) № 37/2010 of 22 December 2009 on pharmacologically active substances and their classification regarding maximum residue limits in foodstuffs of animal origin. Off. J. of the European Union, 2010, no. L15, pp. 1-72. doi: org/10.3000/17252555.L_2010.015.eng.
Hepperle J., Mack D., Sigalov I., Schüler, S., Anastassiades, M. Analysis of ‘‘Amitraz (sum)’’ in pears with incurred residues – Comparison of the approach covering the individual metabolites via LC–MS/MS with the approach involving cleavage to 2,4-dimethylaniline. Food Chem., 2015, vol. 166, pp. 240-247. doi: 10.1016/j.foodchem.2014.06.003.
Ohba Y., Nakajima T., Kanda M., Hayashi H., Matsushima Y., Nakagawa Y., Koike H., Nagano C., Sekimura K., Otsuka K., Sasamoto T., Hashimoto T. Simultaneous determination of nine acaricides and two metabolites in comb honey by LC/MS/MS. Food Addit. Contam. A, 2018, vol. 35, no. 12, pp. 2375-2386. doi: org/10.1080/19440049.2018.1539252.
Kiljanek T., Niewiadowska A., Semeniuk S., Gaweł M., Borzęcka M., Posyniak A. Multi-residue method for the determination of pesticides and pesticide metabolites in honeybees by liquid and gas chromatography coupled with tandem mass spectrometry – Honeybee poisoning incidents. J. Chromatogr. А, 2016, vol. 1435, pp. 100–114. doi: 10.1016/j.chroma.2016.01.045.
GOST 34889-2022. Med natural'nyi. Opredelenie massovoi doli insektitsidov metodom gazovoi khromatografii s mass-spektrometricheskim detektirovaniem [State Standard 34889-2022. Natural Honey. Determination of insecticides by gas chromatography/mass spectrometry]. Minsk, Euro-Asian Council for Standardization, Metrology and Certification, 2022. 11 p. (in Russian).
Gao X., Tan Y., Guo H. Simultaneous determination of amitraz, chlordimeform, formetanate and their main metabolites in human urine by high performance liquid chromatography-tandem mass spectrometry. J. Chromatogr. B, 2017, vol. 1052, pp. 27-33. doi: 10.1016/j.jchromb.2017.03.004.
Guo H., Zhang P., Wang J., Zheng J. Determination of amitraz and its metabolites in whole blood using solid-phase extraction and liquid chromatography–tandem mass spectrometry. J. Chromatogr. B, 2014, vol. 951-952, pp. 89-95. doi: 10.1016/j.jchromb.2014.01.027.
Caldow M., Fussell R. J., Smith F., Sharman M. Development and validation of an analytical method for total amitraz in fruit and honey with quantification by gas chromatography-mass spectrometry. Food Addit. Contam., 2007, vol. 24, no. 3, pp. 280-284. doi: 10.1080/02652030601013638.
Yu H., Tao Y., Le T., Chen D., Ishsan A., Liu Y., Wang, Y., Yuan, Z. Simultaneous determination of amitraz and its metabolite residue in food animal tissues by gas chromatography-electron capture detector and gas chromatography–mass spectrometry with accelerated solvent extraction. J. Chromatogr. B, 2010, vol. 878, no. 21, pp. 1746-1752. https://doi.org/10.1016/j.jchromb.2010.04.034.
Jiménez J.J., Bernal J.L., del Nozal M.J., Alonso C. Extraction and clean-up methods for the determination of amitraz total residues in beeswax by gas chromatography with electron capture detection. Anal. Chim. Acta, 2004, vol. 524, no.1-2, pp. 271-278. doi: 10.1016/j.aca.2004.03.039.
Polonevich A. G., Leschev S. M., Bulhakava V. A., Belyshava L. L. [Development and validation of the method for determination of amitraz in honey using high-performance liquid chromatography with mass spectrometric detection]. Vestsi Natsyyanal’nai akademii navuk Belarusi. Seryya khimichnykh navuk [Proceedings of the National Academy of Sciences of Belarus. Chemical series], 2022, vol. 58, no. 4, pp. 387–397. doi: 10.29235/1561-8331-2022-58-4-387-397. (in Russian).
Polonevich A. G., Leschev S. M., Bulhakava V. A., Belyshava L. L. [Extraction of amitraz and its metabolites with organic solvents]. Vestsi Natsyyanal’nai akademii navuk Belarusi. Seryya khimichnyh navuk [Proceedings of the National Academy of Science of Belarus. Chemical series], 2023, vol. 59, no. 1, pp. 139–149. doi: 10.29235/1561-8331-2023-59-2-139-149. (in Russian).
International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use (ICH), Q2 (R1): Validation of Analytical Procedures: Text and Methodology. Available at: https://database.ich.org/sites/default/files/Q2%28R1%29%20Guideline.pdf.
STB ISO 11095-2009. Kalibrovka lineinaia s ispol'zovaniem standartnykh obraztsov [STB ISO 11095-2009. Linear calibration using reference materials]. Minsk, Belarusian state Institute of Standardization and Certification, 2009. 31 p. (in Russian).
STB ISO 5725-2-2022. Tochnost' (pravil'nost' i pretsizionnost') metodov i rezul'tatov izmerenii. Chast' 2. Osnovnoi metod opredeleniia povtoriaemosti i vosproizvodimosti standartnogo metoda izmerenii [STB ISO 5725-2-2022. Accuracy (trueness and precision) of measurement methods and results. Part 2. Basic method for the determination of repeatability and reproducibility of a standard measurement method]. Minsk, Gosstandart, 2022. 61 p. (in Russian).
Calculation of Measurement Uncertainty in Environmental Laboratories. Nordtest Technical Report 537, 2017. Available at: http://nordtest.info/images/documents/nt-technical-reports/NT_TR_537_edition4_English_Handbook_for_calculation_of_measurement_uncertainty_in_environmental_laboratories.pdf
Analytical quality control and method validation procedures for pesticide residues analysis in food and feed SANTE/12682/2019. Available at: https://www.eurl-pesticides.eu/userfiles/file/EurlALL/AqcGuidance_SANTE_2019_12682.pdf.
Treatment of an observed bias (information leaflet). Available at: https://www.eurachem.org/images/stories/leaflets/mu/bias_01/Eurachem_Bias_Leaflet_01_EN.pdf.
DOI: https://doi.org/10.15826/analitika.2023.27.3.006
Ссылки
- На текущий момент ссылки отсутствуют.
