Crystal structure and oxygen nonstoichiometry of oxides in the Ba-Me-Me’-Y-O (Me, Me’=Co, Fe) system
Abstract
Keywords
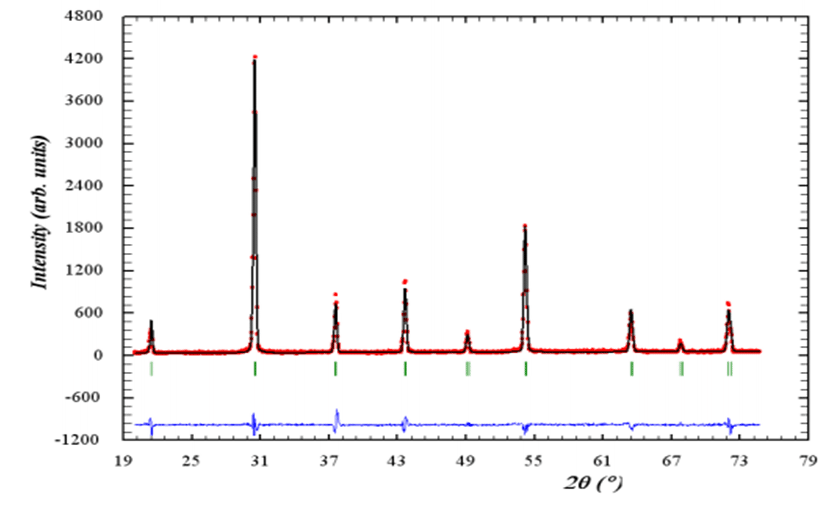
Full Text:
PDFReferences
Lomakov MV, Istomin SYa, Abakumov AM, Van Tendeloo G, Antipov EV. Synthesis and characterization of oxygen-deficient oxides BaCo1 − xYxO3 − y, x = 0.15, 0.25 and 0.33, with the perovskite structure. Solid State Ionics. 2008;179(33-4):1885-9. doi:10.1016/j.ssi.2008.05.004
Shannon RD. Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Acta Cryst. 1976;A32:751–67. doi:10.1107/S0567739476001551
Urusova AS, Cherepanov VA, Aksenova TV, Gavrilova Ya, Kiselev EA. Phase equilibria, crystal structure and oxygen content of intermediate phases in the Y–Ba–Co–O system. J Solid State Chem. 2013;202:207-14. doi:10.1016/j.jssc.2013.03.037
Allred AL, Rochow EG. A scale of electronegativity based on electrostatic force. J Inorg Nucl Chem. 1958;5(4):264–8. doi:10.1016/0022-1902(58)80003-2
DOI: https://doi.org/10.15826/chimtech.2015.2.2.015
Copyright (c) 2015 A. S. Urusova, A. V. Bryuzgina, T. V. Aksenova, V. A. Cherepanov

This work is licensed under a Creative Commons Attribution 4.0 International License.
Chimica Techno Acta, 2014–2025
eISSN 2411-1414
Copyright Notice






