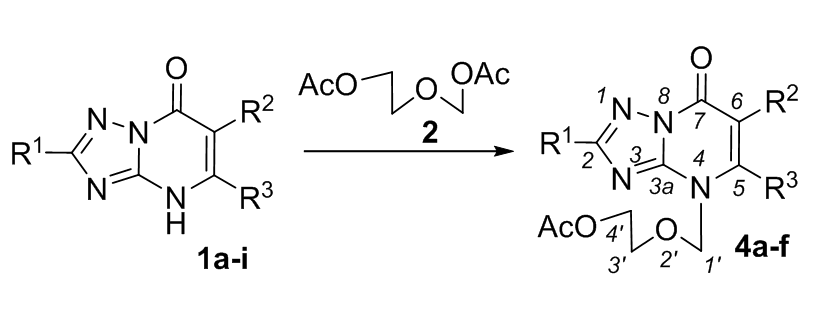
Synthesis of acyclic nucleoside analogues based on 1,2,4-triazolo[1,5-a]pyrimidine-7-ones by one-step Vorbrüggen glycosylation
Abstract
Keywords
Full Text:
PDFReferences
Ai Y, Chen Y, Tang C, Yang GZ, Liang YJ, Fu LW, Liu JC, He HW. Synthesis and in vitro antiproliferative evaluation of pyrimido[5,4-c]quinoline-4-(3H)-one derivatives. Eur J Med Chem. 2012. 47; 206–2013. doi:10.1016/j.ejmech.2011.10.044
Boechat N, Pinheiro LCS, Silva TS, Carvalho AS, Bastos MM, Costa CC, Mendonca JS, Dutra KD, Santos-Filho OA, Pinto AC, Aguiar AC, Ceravolo IP, Krettli AU, Pinheiro S, Valverde AL. New trifluoromethyl triazolopyrimidines as Anti-Plasmodium falciparum agents. Molecules. 2012. 17:7;8285–8302. doi:10.3390/molecules17078285
Sanchez RM, Erhard K, Lin H, Moore ML, Yu H, Luengo JI, Tedesco R, Rivero RA, Hardwicke MA, Plant R, Rominger CM, McSurdy-Freed J, Spengler MD, Raha K, Schaber MD. Synthesis and structure-activity relationships of 1,2,4-triazolo[1,5-a] pyrimidin-7(3H)-ones as novel series of potent β isoform selective phosphatidylinositol 3-kinase inhibitors. Bioorg Med Chem Lett. 2012;22(9):3198–202. doi:10.1016/j.bmcl.2012.03.039
Deev SL, Chupakhin ON, Shestakova TS, Ulomskii EN, Rusinov VL, Yasko MV, Karpenko IL, Korovina AN, Khandazhinskaya AL, Kukhanova MK, Andronova VL, Galegov GA. 1,2,4-Triazoloazine derivatives as a new type of herpes simplex virus inhibitors. Bioorg Chem. 2010. 38:64; 265–270. doi:10.1016/j.bioorg.2010.09.002
DOI: https://doi.org/10.15826/chimtech.2015.2.2.017
Copyright (c) 2015 I. A. Khalymbadzha, S. L. Deev, T. S. Shestakova, V. L. Rusinov, O. N. Chupakhin

This work is licensed under a Creative Commons Attribution 4.0 International License.
Chimica Techno Acta, 2014–2025
eISSN 2411-1414
Copyright Notice






