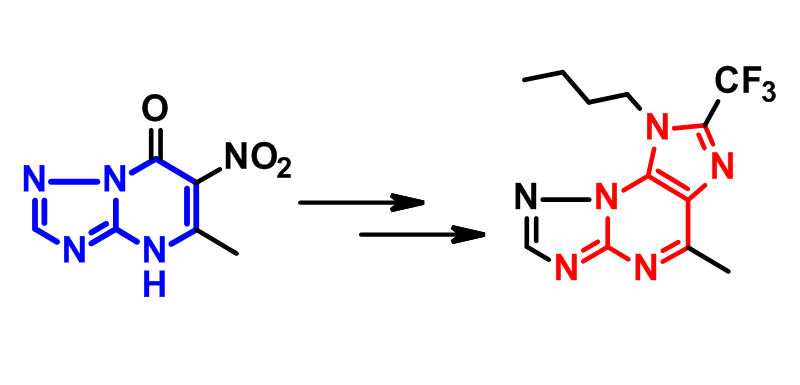
6-Aminotriazolo[1,5-a]pyrimidines as precursors of 1,2,4-triazolo[5,1-b]purines
Abstract
Keywords
Full Text:
PDFReferences
Zhang N, Ayral-Kaloustian S, Nguyen T, Afragola J, Hernandez R, Lucas J, Gibbons J, Beyer C. Synthesis and SAR of [1,2,4]Triazolo[1,5-a]pyrimidines, a Class of Anticancer Agents with a Unique Mechanism of Tubulin Inhibition. J. Med. Chem., 2007;50(2):319–27. doi:10.1021/jm060717i
Zhao XL, Zhao YF, Guo SC, Song HS, Wang D, Gong P. Synthesis and Anti-tumor Activities of Novel [1,2,4]triazolo[1,5-a]pyrimidines. Molecules. 2007;12(5):1136-46. doi:10.3390/12051136
DOI: https://doi.org/10.15826/chimtech.2015.2.2.013
Copyright (c) 2015 K. V. Savateev, S. S. Borisov, E. K. Voinkov, E. N. Ulomsky, V. L. Rusinov, O. N. Chupakhin

This work is licensed under a Creative Commons Attribution 4.0 International License.
Chimica Techno Acta, 2014–2025
eISSN 2411-1414
Copyright Notice






