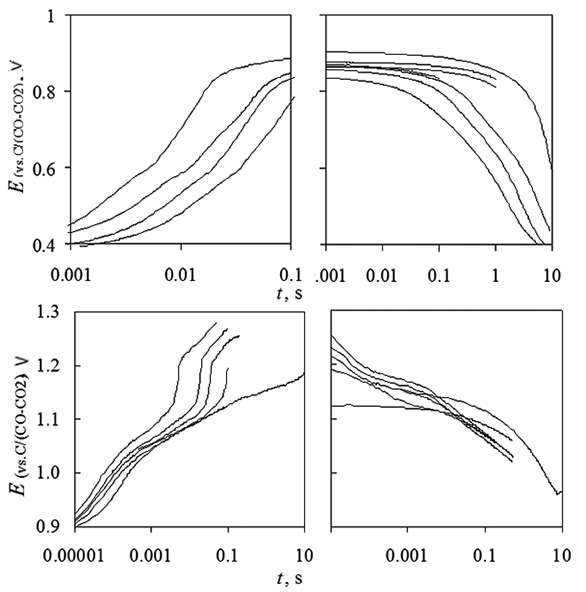
Chronopotentiometry at platinum electrode in KF-NaF-AlF3-Al2O3 melt
Abstract
Keywords
Full Text:
PDFReferences
Nekrasov VN, Suzdaltsev AV, Limanovskaya OV, Khramov AP, Zaikov YuP. Theoretical and experimental study of anode process at the carbon in KF-AlF3-Al2O3 melts. Electrochim Acta. 2012;75:296-304. doi:10.1016/j.electacta.2012.05.007
Yang J, Hryn J N, Davis B R, Roy A, Krumdick G K, Pomykala Jr JA. New opportunities for aluminum electrolysis with metal anodes in a low temperature electrolyte system. TMS Light Metals. 2004:321-6.
Helle S, Pedron M, Assouli B, Davis B, Guay D, Roue L. Structure and high-temperature oxidation behaviour of Cu-Ni-Fe alloys prepared by high-energy ball milling for application as inert anodes in aluminium electrolysis. Corros Sci. 2010;52(10):3348-55. doi:10.1016/j.corsci.2010.06.011
Nekrasov VN, Limanovskaya OV, Suzdaltsev AV, Khramov AP, Zaikov YuP. Stationary anodic process at platinum in KF-NaF-AlF3-Al2O3 melts. Metally. 2014;2014(8):664-70. doi:10.1134/S0036029514080084
Suzdaltsev AV, Khramov AP, Zaikov YuP. Carbon electrode for electrochemical studies in electrolite-alumina melts at 700-960 C. Russ J Electrochem. 2012;48(12):1141-52. doi:10.1134/S1023193512120117
Livingstone S. The Chemistry of Ruthenium, Rhodium, Palladium, Osmium, Iridium and Platinum. Oxford: Pergamon; 1973. 222 p.
Dewing EW, Van der Kouwe E. Anodic Phenomena in Cryolite-Alumina Melts II. Chronopotentiometry at Gold and Platinum Anodes. J Electrochem Soc. 1977;124(1):58-64. doi:10.1149/1.2133245
Bard AJ, Faulkner LR. Electrochemical Methods: Fundamentals and Applications. 2nd ed. New York: John Wiley & Sons; 2001. 833 p.
DOI: https://doi.org/10.15826/chimtech.2015.2.3.020
Copyright (c) 2015 A. V. Suzdaltsev, A. P. Khramov, Yu. P. Zaikov, O. V. Limanovskaya, V. N. Nekrasov

This work is licensed under a Creative Commons Attribution 4.0 International License.
Chimica Techno Acta, 2014–2025
eISSN 2411-1414
Copyright Notice







