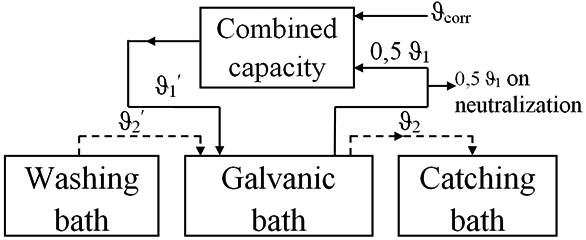
Simulation of an electrolytic bath for electrodeposition of multilayer shielding coatings
Abstract
Keywords
Full Text:
PDFReferences
Rudoi VM, Ostanina TN, Darintseva AB, Ostanin NI, Alikhanova IA, Demakov SL, Prokof'eva AS. Electrodeposition of copper on metal-filled composite support. Russ J Electrochem. 2010;46(6):702–6. doi:10.1134/S1023193510060157
Ovchnnikova SN, Poddubnyi NP, Masliy AI, Boldyrev VV. Schwazacher W. Mutual influence of electrode processes during electrodeposition of layered structures by the single-bath method: The effect of nickel deposition and hydrogen evolution on the transport of copper ions in acetate and sulfamate electrolytes. Russ J Electrochem. 2002;38(11):1210–16. doi:10.1023/A:1021153827310
DOI: https://doi.org/10.15826/chimtech.2015.2.3.021
Copyright (c) 2015 E. A. Dolmatova, A. D. Dayanov, T. N. Ostanina

This work is licensed under a Creative Commons Attribution 4.0 International License.
Chimica Techno Acta, 2014–2025
eISSN 2411-1414
Copyright Notice






