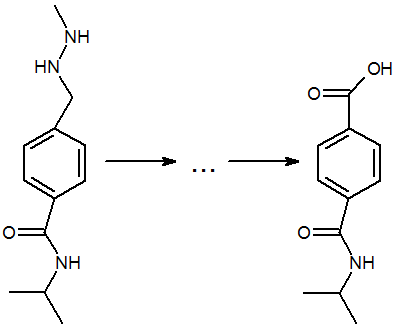
The mechanism for the electrooxidation of procarbazine pharmaceutical preparation in alkaline media and its mathematical description
Abstract
Keywords
Full Text:
PDFReferences
Newton HB, editor. Handbook of Brain Tumor Chemotherapy. Amsterdam: Elsevier; c2006. 586 p. ISBN: 978-0-12-088410-0.
Shiba DA, Weinkam RJ. The in vivo cytotoxic activity of procarbazine and procarbazine metabolites against L1210 ascites leukemia cells in CDF1 mice and the effects of pretreatment with procarbazine, phenobarbital, diphenylhydantoin, and methylprednisolone upon in vivo procarbazine activity. Cancer Chemoth Pharm. 1983;11(2):124-9. doi:10.1007/BF00254261
Aronson JK, editor. Meyler’s Side Effects of Drugs in Cancer and Immunology. Amsterdam: Elsevier Science; 2010. 780 p. ISBN: 9780444532671.
Oliveira SCB, Mendes CHS, Filho FFS, Queiroz NL, Nascimento JAM, Nascimento VB. Electrochemical oxidation mechanism of procarbazine at glassy carbon electrode. J Electroanal Chem. 2015;746(1):51-6. doi:10.1016/j.jelechem.2015.02.033
Mahanthesha KR, Swamy BEK, Pai KV. Poly (alizarin) modified glassy carbon electrode for the electrochemical investigation of Omeprazole: A voltammetric study. Anal Bioanal Electrochem [Internet]. 2014[cited 2016];6(2):234-44. Available from: http://www.abechem.com/No.%202-2014/2014,%206%20(2)%20234-244.pdf
Ojani R, Raoof J, Ahmady A, Hosseini SR. Highly improved methanol oxidation onto carbon paste electrode modified by nickel particles dispersed into poly (2,5-dimethylaniline) film. Caspian Journal of Chemistry [Internet]. 2013[cited 2016];2:45-57. Available from: http://caschemistry.journals.umz.ac.ir/article_510_13d7ba627fde246025b83b64cbda0a4f.pdf
Raoof J, Ojani R, Hosseini SR. An electrochemical investigation of methanol oxidation on nickel hydroxide nanoparticles. South African Journal of Chemistry [Internet]. 2013[cited 2016];66:47-53. Available from: https://www.ajol.info/index.php/sajc/article/download/123096/112636
Ojani R, Raoof JB, Zavvarmahalleh SRH. Electrocatalytic oxidation of methanol on carbon paste electrode modified by nickel ions dispersed into poly (1,5-diaminonaphthalene) film. Electrochim Acta. 2008;53(5):2402-7. doi:10.1016/j.electacta.2007.10.004
Shu H. Applications of poly(3-hexylthiophene) thin film as a hydrazine-sensitive chemoresistor [master's thesis]. [Alabama]: Auburn University; 2006. 112 p.
Tosar JP. Estudio de la inmovilizacion de oligonucleotidos a electrodos modificados de oro:polipirrol, y deteccion electroquimica de secuencias complementarias [master's thesis]. Montevideo (Uruguay): Universidad de la Republica; 2008. Spanish.
Pagitsas M, Sazou D. The improved Franck-FitzHugh model for the electrodissolution of iron in sulphuric acid solutions: linear stability and bifurcation analysis. Derivation of the kinetic equations for the forced Franck-FitzHugh model. Electrochim Acta. 1991;36(8):1301-8. doi:10.1016/0013-4686(91)80009-W
Pearlstein AJ, Johnson JA. Global and Conditional Stability of the Steady and Periodic Solutions of the Franck-FitzHugh Model of Electrodissolution of Fe in H2SO4. J Electrochem Soc. 1989;136(5):1290-9. doi:10.1149/1.2096909
Das I, Agrawal NR, Ansari SA, Gupta SK. Pattern formation and oscillatory electropolymerization of thiophene. Indian J Chem, Sect A: Inorg, Bio-inorg, Phys, Theor Anal Chem. 2008;47(12):1798-803.
Rahman SU, Ba-Shammakh MS. Thermal effects on the process of electropolymerization of pyrrole on mild steel. Synth Met. 2004;140(2-3):207-23. doi:10.1016/S0379-6779(03)00369-2
Liu AS, Oliveira MAS. Electrodeposition of polypyrrole films on aluminum from tartrate aqueous solution. J Braz Chem Soc. 2007;18(1):143-52. doi:10.1590/S0103-50532007000100016
Sazou D. The dynamical behavior of the electrochemical polymerization of indole on Fe in acetonitrile-water mixtures. Synth Met. 2002;130(1):45-54. doi:10.1016/S0379-6779(02)00110-8
McCue JP, Kennedy JH. Oxidation of procarbazine in the presence of Ti(IV). Bioinorg Chem. 1977;7(1):5-21. doi:10.1016/S0006-3061(00)80125-8
Tkach V, Swamy BK, Ojani R, Blanes M, Yagodynets P. El Mecanismo de la Oxidación de Omeprazol Sobre el Electrodo de Carbono Vitroso, Modificado por Polializarina, y Su Descripción Matemática. Orbital: Electron J Chem. 2015;7(1):1-4. Spanish. doi:10.17807/orbital.v7i1.599
Tkach VV, Ojani R, Nechyporuk VV, Yagodynets PI. O estudo matemático do desempenho do sensor eletroquímico de nitrito, baseado em poli(p-aminoacetanilida). Rev Fac Ing, Univ Cent Venez [Internet]. 2015[cited 2016];30(1):181-8. Portuguese. Available from: http://www.scielo.org.ve/pdf/rfiucv/v30n1/art18.pdf
Tkach V, Nechyporuk V, Yagodynets P. The mathematical description for the electropolymerization of furan, pyrrole and thiophene derivatives in alkaline media. Mediterr J Chem [Internet]. 2015[cited 2016];3(6):1122-8. Available from: http://www.medjchem.com/index.php/medjchem/article/download/185/pdf_6
Tkach VV, Nechyporuk VV, Yagodynets PI, Kushnir VM. Descripción matemática de la electropolimerización de monómeros modificados electroquímicamente. Afinidad [Internet]. 2015[cited 2016];72(572):218-22. Spanish. Available from: http://www.raco.cat/index.php/afinidad/article/download/300919/390366
DOI: https://doi.org/10.15826/chimtech.2016.3.1.007
Copyright (c) 2016 V. V. Tkach, S. C. de Oliveira, S. K. B. de Oliveira, R. Ojani, O. V. Elenich, P. I. Yagodynets

This work is licensed under a Creative Commons Attribution 4.0 International License.
Chimica Techno Acta, 2014–2025
eISSN 2411-1414
Copyright Notice






