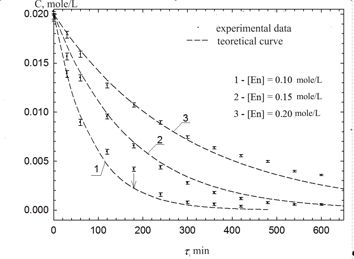
Kinetics of chemical bath deposition of zinc sulfide ZnS
Abstract
Keywords
Full Text:
PDFReferences
Monroy E, Omnes F, Calle F. Wide-bandgap semiconductor ultraviolet photodetectors. Semicond Sci Technol. 2003;18(4):R33-R55. doi:10.1088/0268-1242/18/4/201
Kroeger FA, Hellingman JE. Chemical proof of the presence of chlorine in blue fluorescent zinc sulfide. J Electrochem Soc. 1949;95(2):68-9. doi:10.1149/1.2776738
Chen R, Lockwood DJ. Developments in luminescence and display materials over the last 100 years as reflected in electrochemical society publications. J Electrochem Soc. 2002;149(9):S69-S78. doi:10.1149/1.1502258
Quan Z, Yang D, Li C, Kong D, Yang P, Cheng Z, Lin J. Multicolor tuning of manganese-doped ZnS colloidal nanocrystals. Langmuir. 2009;25(17):10259-62. doi:10.1021/la901056d
Peng WQ, Cong GW, Qu SC, Wan ZG. Synthesis and photoluminescence of ZnS:Cu nanoparticles. Optic Mater. 2006;29(2-3):313-7. doi:10.1016/j.optmat.2005.10.003
Debenham M. Refractive indices of zinc sulfide in the 0.405–13-μm wavelength range. Appl Opt 1984;23(14):2238-9. doi:10.1364/AO.23.002238
Park W, King JS, Neff CW, Liddell C, Summers CJ. ZnS-Based photonic crystals. Phys Stat Sol B. 2002;229(2):949-60. doi:10.1002/1521-3951(200201)229:2<949::AID-PSSB949>3.0.CO;2-K
Nanda J, Sapra S, Sarma DD. Size-selected zinc sulfide nanocrystallites: synthesis, structure, and optical studies. Chem Mater. 2000;12(4):1018-24. doi:10.1021/cm990583f
Zhao Y, Zhang Y, Zhu H, Hadjipanayis GC, Xiao JQ. Low-temperature synthesis of hexagonal (wurtzite) ZnS nanocrystals. J Am Chem Soc. 2004;126(22):6874-5. doi:10.1021/ja048650g
Del'mon B. Kinetika geterogennikh reaktsiy [Kinetics of heterogeneous reactions]. Bazhina NM, Malygina EG, Berdnikova VM, translators; Boldyrev VV, editor. Moscow: Mir; 1972. 556 p. Russian.
Kitaev GA, Uritskaya AA, Yatlova LE, Mirolyubov VP. Deposition of zinc sulfide from a solution of N-allylthiourea. Russ J Appl Chem. 1994; 67(10):1612-5.
Uritskaya AA, Kitaev GA, Belova NS. Kinetics of cadmium sulfide precipitation from aqueous thiourea solutions. Russ J Appl Chem. 2002;75(5):846-848. doi:10.1023/A:1020391419595
MatWeb: Material property data: Data sheets for over 87,000 metals, plastics, ceramics, and composites [Internet]. [Blacksburg (VA)]: Mat-Web LLC, c1996-2011. Available from: http://www.matweb.com/
Haimova IM, Atlava LE, Uritskaya AA, Kitaev GA. [Investigation of kinetics of chemical vapor deposition of zinc sulfide from solutions of allithiourea]. VINITI. Dep. № 859-XII-88. Russian.
Zakhar'evskiy MS. Kinetika i kataliz [Kinetics and catalysis]. Leningrad (USSR): Izdatel'stvo Leningradskogo universiteta [Leningrad University publishing house]; 1963. 314 p. Russian.
DOI: https://doi.org/10.15826/chimtech.2016.3.2.009
Copyright (c) 2016 A. A. Uritskaya, N. S. Kozhevnikova, T. V. Yakubova

This work is licensed under a Creative Commons Attribution 4.0 International License.
Chimica Techno Acta, 2014–2025
eISSN 2411-1414
Copyright Notice






