Combinatorial approach to the synthesis of substituted 1,2,4-triazines
Abstract
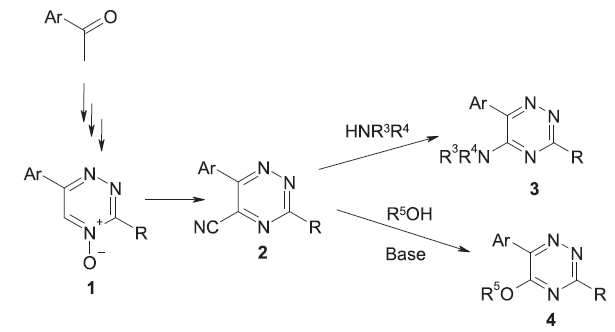
This article describes a convenient and easy method of substitution of the
nitrile group in 5-cyano-1,2,4-triazines with the aim of obtaining of libraries of
substituted 1,2,4-triazines (about 500 compounds).
Keywords
Full Text:
PDFReferences
Huang JJ. Synthesis of fused 1,2,4-triazines: 6- and 7-azapteridine and 6-azapurine ring systems. J Org Chem. 1985;50(13):2293–8. doi:10.1021/jo00213a019
Rykowski A, Branowska D, Makosza M, Ly VP. Reactions of 1,2,4-triazines with nitromethide ion. A convenient method of preparation of 1,2,4-triazin-5-ylcarbaldehyde oximes and their synthetic applications. J Heterocycl Chem. 1996;33(6):1567–71. doi:10.1002/jhet.5570330603
Kozhevnikov VN, Kozhevnikov DN, Shabunina OV, Kataeva NN, Yushchuk SA, Rusinov VL, Chupakhin ON. From 3-chloromethyl-1,2,4-triazine 4-oxides to various substituted pyridines and 1,2,4-triazines. Russ Chem Bull. 2005;54(9):2187–96. doi:10.1007/s11172-006-0095-4
Kozhevnikov DN, Kozhevnikov VN, Kovalev IS, Rusinov VL, Chupakhin ON, Aleksandrov GG. Transformations of 1,2,4-Triazines in Reactions with Nucleophiles: V. SNH and ipso-Substitution in the Synthesis and Transformations of 5-Cyano-1,2,4-triazines. Russ J Org Chem. 2002;38(5):744–50. doi:10.1023/A:1019631610505
Prokhorov AM, Kozhevnikov DN, Rusinov VL, Matern AI, Nikitin MM, Chupakhin ON, Eremenko IL, Aleksandrov GG. 5-Acylmethyl-3-(2-pyridyl)-1,2,4-triazines: Synthesis and Complexes with Cu(II). Russ J Org Chem. 2005;41(11):1702–5. doi:10.1007/s11178-006-0022-z
Kopchuk DS, Chepchugov NV, Kovalev IS, Santra S, Rahman M, Giri K, Zyryanov GV, Majee A, Charushin VN, Chupakhin ON. Solvent-free synthesis of 5-(aryl/alkyl)amino-1,2,4-triazines and α-arylamino-2,2′-bipyridines with greener prospects. RSC Adv. 2017;7:9610–19. doi:10.1039/C6RA26305D
DOI: https://doi.org/10.15826/chimtech.2017.4.1.021
Copyright (c) 2017 D. N. Коzshevnikov, V. N. Коzshevnikov

This work is licensed under a Creative Commons Attribution 4.0 International License.
Chimica Techno Acta, 2014–2025
eISSN 2411-1414
Copyright Notice






