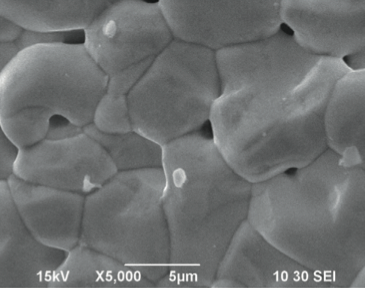
Preparation and characterization of Bi26-2xMn2xMo10O69-d and Bi26.4Mn0.6Mo10-2yMe2yO69-d (Me = V, Fe) Solid Solutions
Abstract
Keywords
Full Text:
PDFReferences
Boivin JC. Structural and electrochemical features of oxide ion conductors. Int J Inorg Mat. 2001;3:1261-6. doi:10.1016/s1466-6049(01)00118-0.
Abraham F, Boivin JC, Mairesse G, Nowogrocki G. The bimevox series: A new family of high performances oxide ion conductors. Solid State Ionics. 1990;40-1:934-7. DOI:10.1016/0167-2738(90)90157-M.
Fonseca FC, Steil MC, Vannier RN, Mairesse G, Muccillo R. Grain-sized influence on the phase transition of Bi26Mo9WO69: an X-ray diffraction and impedance spectroscopy study. Solid State Ionics. 2001;140:161-71. DOI:10.1016/S0167-2738(01)00705-6.
Buttrey DJ, Vogt T, Yap GPA, Rheingold AL. The structure of Bi26Mo10O69. Mater Res Bull. 1997;32:947-62. DOI:10.1016/s0025-5408(97)00063-9.
Vannier RN, Mairesse G, Abraham F, Nowogorski G. Bi26Mo10Oδ solid solution type in the Bi2O3–MoO3–V2O5 ternary diagram. J Solid State Chem. 1996;122:394-406. DOI:10.1006/jssc.1996.0133.
Vannier RN, Danze S, Nowogrocki G, Huve M, Mairesse G. A new class of mono-dimensional bismuth-based oxide anion conductors with a structure based on [Bi12O14]∞ columns. Solid State Ionics. 2000;136-7:51-9. DOI:10.1016/S0167-2738(00)00351-9.
Ling CD, Miiller W, Johnson MR, Richard D, Rols S, Madge J, Evans IR. Local structure, dynamics, and the mechanisms of oxide ionic conduction in Bi26Mo10O69. Chem Mater. 2012;24:4607-14. DOI:10.1021/cm303202r.
Bastide B, Enjalbert R, Salles P, Galy J. Ionic conductivity of the oxide family Bi[Bi12O14][(Mo,M)O4]5 with M=Li, Mg, Al, Si, Ge and V. Solid State Ionics. 2003;158:351-8. doi:10.1016/s0167-2738(02)00910-4.
Mikhailovskaya ZA, Buyanova ES, Petrova SA, Morozova MV, Zhukovskiy VM, Zakharov RG, Tarakina NV, Berger IF. Cobalt-doped Bi26Mo10O69 : crystal structure and conductivity. J Solid State Chem. 2013;204:9-15. DOI: 10.1016/j.jssc.2013.05.006.
Enjalbert R, Hasselmann G, Galy J. A new mixed oxide with (Bi12O14)n columns: PbBi12Mo5O34. Acta Crystallogr, Sect C: Struct Chem. 1997;53:269-72. DOI:10.1107/s0108270196013698.
Galy J, Salles P, Rozier P, Castro A. Anionic conductors Ln2/3[Bi12O14](MoO4)5 with Ln=La, Nd, Gd, Ho, Yb. Synthesis–spark plasma sintering–structure–electric properties. Solid State Ionics. 2006;177:2897-902. DOI:10.1016/j.ssi.2006.07.059.
Mikhaylovskaya ZA, Buyanova ES, Morozova MV, Petrova SA, Nikolaenko IV. Mn-doped Bi26Mo10O69-d: synthesis and characterization. Ionics. 2017;23:1107-14. DOI:10.1007/s11581-016-1917-5.
Mikhaylovskaya ZA, Morozova MV, Buyanova ES, Petrova SA Abrahams I. Iron-doped Bi26Mo10O69 bismuth molybdate:synthesis, properties and structure. In: Abstract Book of the 11th international symposium on systems with fast ionic transport. 2014 Jun 25-29; Gdańsk University of Technology, Gdańsk-Sobieszewo, Poland. 2014. P. 78.
Irvine JTS, Sinclair DC, West AR. Electroceramics: Characterization by impedance spectroscopy. Adv Mater. 1990;2:132-8. DOI:10.1002/adma.19900020304.
DOI: https://doi.org/10.15826/chimtech/2017.4.2.027
Copyright (c) 2017 Z. A. Mikhaylovskaya, M. V. Morozova, E. S. Buyanova, S. A. Petrova, I. V. Nikolaenko, D. G. Kellerman

This work is licensed under a Creative Commons Attribution 4.0 International License.
Chimica Techno Acta, 2014–2025
eISSN 2411-1414
Copyright Notice






