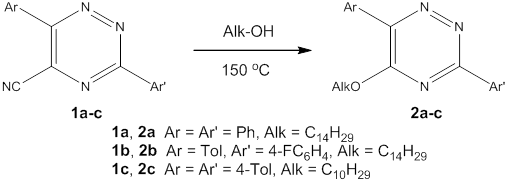
The synthesis of 1,2,4-triazines bearing the residues of higher alcohols in the 5-position via the ipso-substitution of cyano group under the solvent-free conditions
Abstract
Keywords
Full Text:
PDFReferences
Ueno Y, Morishita K, Muraoka M, Ohashi N, inventors; Sumitomo Pharmaceuticals Company Limited, assignee. LDL receptor gene expression promoters. United States patent US6,159,974 A. 2000 Dec 12.
Lewis RT, Jones P, Petrocchi A, Reyna N, Hamilton MM, Leo E, inventors; Board of Regents, University of Texas System, assignee. Mth1 inhibitors for treating disease. World patent WO 2016145383 A1. 2016 Sep 15.
Ple N, Turck A, Queguiner G, Glassl B, Neunhoeffer H. Chemistry of 1,2,4-triazines, XV. First metalation of 1,2,4-triazine derivatives. Liebigs Ann Chem. 1993;6:583–5. DOI:10.1002/jlac.199319930195.
Neunhoeffer H, Reichel D. [1,2,4]Triazino[6,5-e]-1,2,4-triazine; II. Synthesis. 1988;11:877–9. DOI:10.1055/s-1988–27734.
Katagiri N, Watanabe H, Kaneko C. Cycloadditions in Syntheses. XXXVI: Syntheses of 6-Trifluoromethyl-1, 2, 4-triazines and –1, 2, 4-triazin-5-ones and Their Pericyclic Reactions with Olefins. Chem Pharm Bull. 1988;36:3354–72. DOI:10.1248/cpb.36.3354.
Jacobsen NW, Rose SE. 1,2,4-Triazines. II. New Zwitterionic Methylation Products of Some 1,2,4-Triazin-5(2H)-Ones and Their Identification by Carbon-13 Nuclear Magnetic Resonance Spectroscopy. Aust J Chem. 1987;40(5):967–75. DOI:10.1071/CH9870967.
Hoornaert G JC, Kilonda A, Heeres J, Lewi PJ, de Jonge MR, Daeyaert FFD, Vinkers HM, Koymans LMH, Janssen PAJ, inventors; Janssen Pharmaceutica NV, Xavier F, Herwig J, assignee. HIV inhibiting 1,2,4-triazin-6-one derivatives. World Patent WO2006015985 A1. 2006 Feb 16.
Neunhoeffer H, Reichel D, Cullmann B, Rehn I. Zur Chemie der 1,2,4-Triazine, XIV. Synthese und Reaktionen von 5-Chlor-1,2,4-triazinen. Liebigs Ann Chem. 1990;7:631–40. German. DOI:10.1002/jlac.1990199001121.
Konno S, Ohba S, Agata M, Aizawa Y, Sagi M, Yamanaka H. Studies on as-Triazine Derivatives. VIII. Synthesis of 5-Substituted 1,2,4-Triazines. Heterocycles. 1987;26(12):3259–64. DOI:10.3987/R-1987–12–3259.
Konno S, Sagi M, Yokoyama M, Yamanaka H. Studies on as-Triazine Derivatives. XVII. Chlorination of 5,6-Dimethyl-3-phenyl-as-triazine. Heterocycles. 1990;31(11):1933–5. DOI:10.3987/COM-90–5585.
Dudfield P J, Le VD, Lindell SD, Rees CW. Synthesis of C-ribosyl imidazo[2,1-f][1,2,4]triazines as inhibitors of adenosine and AMP deaminases. J Chem Soc, Perkin Trans. 1. 1999:2929–36. DOI:10.1039/A904065J.
Barlow MG, Haszeldine RN, Simon C, Simpkin DJ, Ziervogel G. Heterocyclic polyfluoro-compounds. Part 39. Preparation and some nucleophilic substitution reactions of trifluoro-1,2,4-triazine. J Chem Soc, Perkin Trans. 1. 1982:1251–4. DOI:10.1039/P19820001251.
Chupakhin ON, Rusinov VL, Ulomsky EN, Kojevnikov DN, Neunhoeffer H. Nucleophilic substitution of hydrogen in the reaction of 1,2,4-triazine-4-oxides with cyanides. Mendeleev Commun. 1997;7(2):66–7. DOI:10.1070/MC1997v007n02ABEH000700.
Kozhevnikov DN. Sintez funktsionalizirovannykh azageterotsiklov na osnove reaktsiy nukleofil’nogo zameshcheniya vodoroda v ryadu N-oksidov azinov [dissertation]. Ekaterinburg (Russia): Ural’skiy gosudarstvennyy tekhnicheskiy universitet-UPI; 2004. 259 p. (In Russian.)
Kozhevnikov DN, Kozhevnikov VN, Kovalev IS, Rusinov VL, Chupakhin ON, Aleksandrov GG. Transformations of 1,2,4-Triazines in Reactions with Nucleophiles: V. SNH and ipso-Substitution in the Synthesis and Transformations of 5-Cyano-1,2,4-triazines. Russ J Org Chem. 2002;38(5):744–50. DOI:10.1023/A:1019631610505.
Huang JJ. Synthesis of fused 1,2,4-triazines: 6- and 7-azapteridine and 6-azapurine ring systems. J Org Chem. 1985;50(13):2293–8. DOI:10.1021/jo00213a019.
Huang JJ. Nucleophilic substitution of 1,2,4-triazines. J Heterocycl Chem. 1985;22(5):1329–32. DOI:10.1002/jhet.5570220537.
Rykowski A, Branowska D, Makosza M, Van Ly P. Reactions of 1,2,4-triazines with nitromethide ion. A convenient method of preparation of 1,2,4-triazin-5-ylcarbaldehyde oximes and their synthetic applications. J Heterocycl Chem. 1996;33(6):1567–71. DOI:10.1002/jhet.5570330603.
Nikitina TV, Kozhevnikov DN, Rusinov VL, Chupakhin ON. Vestnik Ural’skogo gosudarstvennogo tekhnicheskogo universiteta – UPI, Seriya Khimiya. 2003;3:79–81. Russian.
Prokhorov AM, Shumkov AA, Ustinova MM, Kozhevnikov DN, Rusinov VL, Chupakhin ON. Vestnik Ural’skogo gosudarstvennogo tekhnicheskogo universiteta – UPI, Seriya Khimiya. 2003;3:82–4. Russian.
Kozshevnikov DN, Kozshevnikov VN. Combinatorial approach to the synthesis of substituted 1,2,4-triazines. Chimica Techo Acta. 2016;4(1):25–8. DOI:10.15826/chimtech.2017.4.1.021.
Kopchuk DS, Chepchugov NV, Kovalev IS, Santra S, Rahman M, Giri K, Zyryanov GV, Majee A, Charushin VN, Chupakhin ON. Solvent-free synthesis of 5-(aryl/alkyl)amino-1,2,4-triazines and α-arylamino-2,2'-bipyridines with greener prospects. RSC Adv. 2017;7(16):9610–9. DOI:10.1039/c6ra26305d.
Kopchuk DS, Chepchugov NV, Taniya OS, Khasanov AF, Giri K, Kovalev IS, Santra S, Zyryanov GV, Majee A, Rusinov VL, Chupakhin ON. 3-Cyano-2-azaanthracene-based «push-pull» fluorophores: A one-step preparation from 5-cyano-1,2,4-triazines and 2,3-dehydronaphthalene, generated in situ. Tetrahedron Lett. 2016;57(50):5639–43. DOI:10.1016/j.tetlet.2016.11.008.
DOI: https://doi.org/10.15826/chimtech.2017.4.2.026
Copyright (c) 2017 A. P. Krinochkin, D. S. Kopchuk, E. S. Starnovskaya, Ya. K. Shtaiz, A. F. Khasanov, I. S. Kovalev, O. S. Taniya, G. V. Zyryanov, V. L. Rusinov, O. N. Chupakhin

This work is licensed under a Creative Commons Attribution 4.0 International License.
Chimica Techno Acta, 2014–2025
eISSN 2411-1414
Copyright Notice






