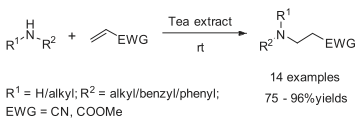
Conjugated Addition of Amines to Electron Deficient Alkenes: A Green Approach
Abstract
Keywords
Full Text:
PDFReferences
Permutter P. Conjugated Addition Reactions in Organic Synthesis. Oxford: Pergamon Press; 1992. 373 p.
Bartoli G, Cimarelli C, Marcantoni E, Palmieri G, Petrini M. Chemo- and diastereoselective reduction of β-enamino esters: A convenient synthesis of both cis- and trans-γ-amino alcohols and β-amino esters. J Org Chem. 1994;59(18):5328-35. doi:10.1021/jo00097a039
Elango S, Yan TH. A short synthesis of (+)-narciclasine via a strategy derived from stereocontrolled epoxide formation and SnCl4-catalyzed arene-epoxide coupling.J Org Chem. 2002;67(20):6954-9. doi:10.1021/jo020155k
Elango S, Yan TH. A short synthesis of (+)-lycoricidine. Tetrahedron. 2002;58(36):7335-8. doi:10.1016/S0040-4020(02)00736-6
Banik BK, Becker FF, Banik I. Synthesis of anticancer β-lactams: Mechanism of action. Bioorg Med Chem. 2004;12(10):2523-8. doi:10.1016/j.bmc.2004.03.033
Graul A, Castaner J. Atorvastatin calcium. Hypolipidemic HMG-CoA reductase inhibitor. Drugs Future. 1997;22(9):956. PMID:9399600.
Ishitani H, Ueno M, Kobayashi S. Enantioselective mannich-type reactions using a novel chiral zirconium catalyst for the synthesis of optically active β-amino acid derivatives. J Am Chem Soc. 2000;122(34):8180-6. doi:10.1021/ja001642p
Jenner G. Catalytic high pressure synthesis of hindered β-aminoesters. Tetrahedron Lett. 1995;36(2):233-6. doi:10.1016/0040-4039(94)02215-W
D’Angelo J, Maddaluno J. Enantioselective Synthesis of B-Amino Esters through High-Pressure-Induced Addition of Amines to A, B-Ethylenic Esters. J Am Chem Soc. 1986;108(25):8112-4. doi:10.1021/ja00285a051
Matsubara S, Yoshiyoka M, Utimoto K. Lanthanoid Triflate Catalyzed Conjugate Addition of Amines to α, β-Unsaturated Esters. A Facile Route to Optically Active β-Lactam. Chem Lett. 1994;23(5):827-30. doi:10.1246/cl.1994.827
Bartoli G, Bartolacci M, Giuliani A, Marcantoni E, Massimo M, Torregiani E. Improved heteroatom nucleophilic addition to electron-poor alkenes promoted by CeCl3·7H2O/NaI system supported on alumina in solvent-free conditions. J Org Chem. 2005;70(1):169-74. doi:10.1021/jo048329g
Loh TP, Wei LL. Indium trichloride-catalyzed conjugate addition of amines to α,β-ethylenic compounds in water. Synlett. 1998;9:975-6. doi:10.1016/j.tetlet.2005.03.112
Wabnitz TC, Spencer JB. Convenient synthesis of Cbz-protected β-amino ketones by a copper-catalysed conjugate addition reaction. Tetrahedron Lett. 2002;43(21):3891-4. doi:10.1016/S0040-4039(02)00654-8
Xu LW, Li JW, Xia CG, Zhou SL, Hu XX. Efficient Copper-Catalyzed Chemo Selective Conjugate Addition of Aliphatic Amines to α,β-Unsaturated Compounds in Water. Synlett. 2003;25:2425-7. doi:10.1055/s-2003-42125
Duan Z, Xuan X, Li T, Yang C, Wu Y. Cerium(IV) ammonium nitrate (CAN) catalyzed aza-Michael addition of amines to α,β-unsaturated electrophiles. Tetrahedron Lett. 2006;47(31):5433-6. doi:10.1016/j.tetlet.2006.05.182
Yang L, Xu LW, Xia CG. Highly efficient KF/Al2O3-catalyzed versatile hetero-Michael addition of nitrogen, oxygen, and sulfur nucleophiles to α,β-ethylenic compounds. Tetrahedron Lett. 2005;46(19):3279-82. doi:10.1016/j.tetlet.2005.03.112
Shaikh NS, Deshpande VH, Bedekar AV. Clay catalyzed chemoselective Michael type addition of aliphatic amines to α,β-ethylenic compounds. Tetrahedron. 2001;57(43):9045-8. doi:10.1016/S0040-4020(01)00911-5
Azizi N, Saidi MR. LiClO4 accelerated Michael addition of amines to α,β-unsaturated olefins under solvent-free conditions. Tetrahedron. 2004;60(2):383-7. doi:10.1016/j.tet.2003.11.012
Varala R, Alam MM, Adapa SR. Chemoselective Michael type addition of aliphatic amines to α,β-ethylenic compounds using bismuth triflate catalyst. Synlett.2003;5:720-2. doi:10.1055/s-2003-38345
Srivastava N, Banik BK. Bismuth nitrate-catalyzed versatile Michael reactions. J Org Chem. 2003;68(6):2109-14. doi:10.1021/jo026550s
Reboule I, Gil R, Collin J. Aza-Michael reactions catalyzed by samarium diiodide.Tetrahedron Lett. 2005;46(45):7761-4. doi:10.1016/j.tetlet.2005.09.039
Kantam ML, Neeraja V, Kavita B, Neelima B, Chaudhuri MK, Hussain S. Cu(acac)2 immobilized in ionic liquids: A recoverable and reusable catalytic system for aza-Michael reactions. Adv Synth Catal. 2005;347(6):763-6. doi:10.1002/adsc.200404361
Xu, LW, Li JW, Zhou SL, Xia CG. A green, ionic liquid and quaternary ammonium salt-catalyzed aza-Michael reaction of α,β-ethylenic compounds with amines in water. New J Chem. 2004;28(2):183-4. doi:10.1039/b312047c
Karodia N, Liu X, Ludley P, Pletsas D, Stevenson G. The ionic liquid ethyltri-n-butylphosphonium tosylate as solvent for the acid-catalysed hetero-Michael reaction. Tetrahedron. 2006;62(48):11039-43. doi:10.1016/j.tet.2006.09.052
Chaudhuri MK, Hussain S, Kantam ML, Neelima B. Boric acid: A novel and safe catalyst for aza-Michael reactions in water. Tetrahedron Lett. 2005;46(48):8329-31. doi:10.1016/j.tetlet.2005.09.167
Hussain S, Bharadwaj SK, Chaudhuri MK, Kalita H. Borax as an efficient metal-free catalyst for hetero-Michael reactions in an aqueous medium. Eur J Org Chem. 2007;2:374-8. doi:10.1002/ejoc.200600691
Hashemi MM, Eftekhari-Sis B, Abdollahifar A, Khalili B. ZrOCl2·8H2O on montmorillonite K10 accelerated conjugate addition of amines to α,β-unsaturated alkenes under solvent-free conditions. Tetrahedron. 2006;62(4):672-7. doi:10.1016/j.tet.2005.10.006
Surendra K, Krishnaveni NS, Sridhar R, Rao KR. β-Cyclodextrin promoted aza-Michael addition of amines to conjugated alkenes in water.Tetrahedron Lett.2006;47(13):2125-7. doi:10.1016/j.tetlet.2006.01.124
Khan AT, Parvin T, Gazi S, Choudhury LH. Bromodimethylsulfonium bromide mediated Michael addition of amines to electron deficient alkenes. Tetrahedron Lett. 2007;48(22):3805-8. doi:10.1016/j.tetlet.2007.03.163
Fetterly BM, Jana NK, Verkade JG. [HP(HNCH2CH2)3N]NO3: An efficient homogeneous and solid-supported promoter for aza and thia-Michael reactions and for Strecker reactions. Tetrahedron. 2006;62(2-3):440-56. doi:10.1016/j.tet.2005.09.117
Roy A, Kundu D, Kundu SK, Majee A, Hajra A. Manganese (II) chloride-catalyzed conjugated addition of amines to electron deficient alkenes in methanol-water medium. The Open Catalysis Journal.2010;3(1):34-9. doi:10.2174/1876214X01003010034
Ranu BC, Dey SS, Hajra A. Solvent-free, catalyst-free Michael-type addition of amines toelectron-deficient alkenes. ARKIVOC. 2002;7:76-81. doi:10.3998/ark.5550190.0003.709
Ranu BC, Banerjee S. Significant rate acceleration of the aza-Michael reaction in water. Tetrahedron Lett. 2007;48(1):141-3. doi:10.1016/j.tetlet.2006.10.142
Kobayashi S, Manabe K. Development of novel Lewis acid catalysts for selective organic reactions in aqueous media. Acc Chem Res. 2002;35(2):209-17. doi:10.1021/ar000145a
Kobayashi S, Sugiura M, Kitagawa H, Lam W WL. Rare-earth metal triflates in organic synthesis. Chem Rev. 2002;102(6):2227-302. doi:10.1021/cr010289i
Mallikarjuna NN, Varma RS. Green synthesis of silver and palladium nanoparticles at room temperature using coffee and tea extract. Green Chem. 2008;10(8):859-62. doi:10.1039/b804703k
Vuong QV, Golding JB, Stathopoulos CE, Roach PD. Effects of aqueous brewing solution pH on the extraction of the major green tea constituents. Food Res Int.2013;53(2):713-9. doi:10.1016/j.foodres.2012.09.017
Ghosal NC, Santra S, Das S, Hajra A, Zyryanov GV, Majee A. Organocatalysis by an aprotic imidazolium zwitterion: Regioselective ring-opening of aziridines and applicable to gram scale synthesis. Green Chem. 2016;18(2):565-74. doi:10.1039/c5gc01323b
Santra S, Kopchuk DS, Kovalev IS, Zyryanov GV, Majee A, Charushin VN, Chupakhin ON. Solvent-free synthesis of pillar[6]arenes. Green Chem. 2016;18(2):423-6. doi:10.1039/c5gc01505g
Mahato S, Santra S, Chatterjee R, Zyryanov GV, Hajra A, Majee A. Brønsted acidic ionic liquid-catalyzed tandem reaction: an efficient approach towards regioselective synthesis of pyrano[3,2-c]coumarins under solvent-free conditions bearing lower E-factors. Green Chem. Forthcoming 2017. doi:10.1039/c7gc01158j
Santra S, Rahman M, Roy A, Majee A, Hajra A. Nano-indium oxide: An efficient catalyst for one-pot synthesis of 2,3-dihydroquinazolin-4(1H)-ones with a greener prospect. Catal Commun. 2014;49:52-7. doi:10.1016/j.catcom.2014.01.032
DOI: https://doi.org/10.15826/chimtech/2017.4.2.029
Copyright (c) 2017 Anindita Mukherjee, Rana Chatterjee, Aramita De, Satyajit Samanta, Sachinta Mahato, Nirnita Chakraborty Ghosal, G. V. Zyryanov, Adinath Majee

This work is licensed under a Creative Commons Attribution 4.0 International License.
Chimica Techno Acta, 2014–2025
eISSN 2411-1414
Copyright Notice






