Thermodynamic database for multicomponent oxide systems
Abstract
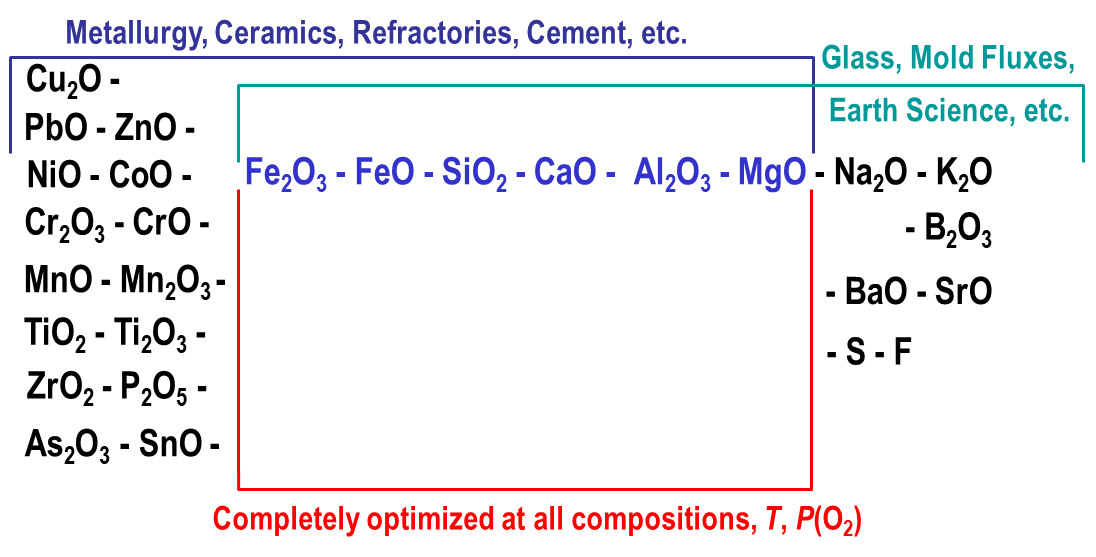
A state-of-the-art thermodynamic database has been developed for multicomponent oxide systems. It can be used in combination with FactSage software to calculate the properties of metallurgical slags, glasses, ceramics, refractories, minerals, cements, etc. The database has been developed by collecting all available structural, thermodynamic, and phase equilibria data for a particular chemical system, critical evaluation of this information, developing a thermodynamic model for each solution phase and optimization of model parameters to reproduce the experimental data. Then the models are used to estimate the thermodynamic properties of multicomponent solutions from the properties of lower-order subsystems. Oxide phases often exhibit complex structures and strong interactions between components, which require more sophisticated models than are normally used, for example, for metal alloys. Short-range ordering is rather common and random mixing is often not a good approximation. The models for multicomponent liquid and solid solutions have been developed within the Modified Quasichemical Formalism and Compound Energy Formalism. Optimized model equations are consistent with thermodynamic principles and fully characterize a chemical system, requiring much less experimental work to achieve this goal since only a few measurements are needed in higher-order systems to validate the model estimates. The database can be readily used in conjunction with the FactSage Gibbs energy minimization software to calculate any stable or metastable phase equilibria and phase diagrams. The present article outlines the major components and phases that are currently available in the oxide database, as well as the most important features of the models that have been developed. The model and database have also been developed for the viscosity of oxide melts and glasses. The model links the viscosity to the structure of the liquid phase, which is estimated using the thermodynamic database.
Keywords
Full Text:
PDFReferences
Andersson JO, Helander T, Hoglund L, Shi P, Sundman B. Thermo-Calc & DICTRA, computational tools for materials science. CALPHAD. 2002;26(2):273-312. doi:10.1016/s0364-5916(02)00037-8
Davies RH, Dinsdale AT, Gisby JA, Robinson JAJ, Martin SM. MTDATA - thermodynamic and phase equilibrium software from the national physical laboratory. CALPHAD. 2002;26(2):229-71. doi:10.1016/s0364-5916(02)00036-6
Cao W, Chen SL, Zhang F, Wu K, Yang Y, Chang YA, et al. PANDAT software with PanEngine, PanOptimizer and PanPrecipitation for multi-component phase diagram calculation and materials property simulation. CALPHAD. 2009;33(2):328-42. doi:10.1016/j.calphad.2008.08.004
Bale CW, Chartrand P, Decterov S, Eriksson G, Hack K, Mahfoud RB, et al. FactSage Thermochemical Software and Databases. CALPHAD. 2002;26(2):189-228. doi:10.1016/S0364-5916(02)00035-4
Bale CW, Belisle E, Chartrand P, Decterov SA, Eriksson G, Gheribi AE, et al. FactSage thermochemical software and databases, 2010-2016. CALPHAD. 2016;54:35-53. doi:10.1016/j.calphad.2016.05.002
Eriksson G, Pelton AD. Critical Evaluation and Optimization of the Thermodynamic Properties and Phase Diagrams of the CaO-Al2O3, Al2O3-SiO2, and CaO-Al2O3-SiO2 Systems. Metall Trans. 1993;24:807-16. doi:10.1007/BF02663141
Jak E, Decterov SA, Hayes PC, Pelton AD. Thermodynamic Modelling of the System Al2O3-SiO2-CaO-FeO-Fe2O3 to Predict the Flux Requirements for Coal Ash Slags. Fuel. 1998;77(1/2):77-84. doi:10.1016/S0016-2361(97)00137-3
Jak E, Decterov SA, Pelton AD, Happ J, Hayes PC. Thermodynamic Modelling of the System Al2O3-SiO2-CaO-FeO-Fe2O3 to Characterise Coal Ash Slags. In: Impact of Mineral Impurities in Solid Fuel Combustion; R.P Gupta TFW, Baxter L, Eds. Kluwer Academic/Plenum Publ., NY: 1999; p. 723-33.
Jak E, Decterov SA, Zhao B, Pelton AD, Hayes PC. Coupled Experimental and Thermodynamic Modelling Studies for Metallurgical Smelting and Coal Combustion Slag Systems. Metall Mater Trans B. 2000;31B(4):621-30. doi:10.1007/s11663-000-0100-5
Jak E, Hayes P, Pelton AD, Decterov SA. Thermodynamic optimisation of the FeO-Fe2O3-SiO2 (Fe-O-Si) system with FactSage. Int J Mater Res. 2007;98(9):847-54. doi:10.3139/146.101541
Decterov SA, Jung I-H, Pelton AD. Thermodynamic Modeling of the FeO-Fe2O3-MgO-SiO2 System. J Am Ceram Soc. 2002;85(12):2903-10. doi:10.1111/j.1151-2916.2002.tb00554.x
Jung I-H, Decterov SA, Pelton AD. Thermodynamic Modeling of the CaO-MgO-Al2O3-SiO2 System. In: TMS Annual Meeting, Computational Phase Transformations; 15-21 February 2002; Seattle, WA, USA: 2002.
Jung I-H. Critical Evaluation and Thermodynamic Modeling of Phase Equilibria in Multicomponent Oxide Systems [PhD thesis]. Montreal (Canada): École Polytechnique de Montréal; 2003. 338 p.
Jung I-H, Decterov SA, Pelton AD. Critical Thermodynamic Evaluation and Optimization of the Fe-Mg-O System. J Phys Chem Solids. 2004;65(10):1683-95. doi:10.1016/j.jpcs.2004.04.005
Jung I-H, Decterov SA, Pelton AD. Critical Thermodynamic Evaluation and Optimization of the MgO-Al2O3, CaO-MgO-Al2O3 and MgO-Al2O3-SiO2 Systems. J Phase Equilib. 2004;25(4):329-45. doi:10.1361/15477030420166
Jung I-H, Decterov SA, Pelton AD. Critical Thermodynamic Evaluation and Optimization of the CaO-MgO-SiO2 System. J Eur Ceram Soc. 2005;25(4):313-33. doi:10.1016/j.jeurceramsoc.2004.02.012
Hidayat T, Shishin D, Decterov SA, Jak E. Thermodynamic optimization of the Ca-Fe-O system. Metall Trans B. 2016;47(1):256-81. doi:10.1007/s11663-015-0501-0
Hidayat T, Shishin D, Decterov SA, Jak E. Critical thermodynamic re-evaluation and re-optimization of the CaO-FeO-Fe2O3-SiO2 system. CALPHAD. 2017;56:58-71. doi:10.1016/j.calphad.2016.11.009
Shishin D, Prostakova V, Jak E, Decterov S. Critical assessment and thermodynamic modeling of the Al–Fe–O system. Metall Mater Trans B. 2016;47(1):397-424. doi:10.1007/s11663-015-0493-9
Hidayat T, Shishin D, Decterov SA, Jak E. Experimental Study and Thermodynamic Re-evaluation of the FeO-Fe2O3-SiO2 System. J Phase Equilib Diffus. 2017;38(4):477-92. doi: 10.1007/s11669-017-0535-x
Decterov SA, Pelton AD. A Thermodynamic Database for Copper Smelting and Converting. Metall Mater Trans B. 1999;30B(4):661-9. doi:10.1007/s11663-999-0027-4
Shishin D, Hidayat T, Jak E, Decterov S. Critical assessment and thermodynamic modeling of Cu-Fe-O system. CALPHAD. 2013;41:160-79. doi:10.1016/j.calphad.2013.04.001
Hidayat T, Jak E. Thermodynamic modeling of the "Cu2O"-SiO2, "Cu2O"-CaO, and "Cu2O"-CaO-SiO2 systems in equilibrium with metallic copper. Int J Mater Res. 2014;105(3):249-57. doi:10.3139/146.111023
Shishin D, Jak E, Decterov SA. Thermodynamic assessment and database for the Cu–Fe–O–S system. CALPHAD. 2015;50:144-60. doi:10.1016/j.calphad.2015.06.004
Hidayat T, Shishin D, Decterov S, Jak E. Critical assessment and thermodynamic modeling of the Cu-Fe-O-Si system. CALPHAD. 2017;58:101-14. doi:10.1016/j.calphad.2017.06.003
Jak E, Zhao B, Hayes PC, Decterov SA, Pelton AD. Coupled Experimental and Thermodynamic Modelling Studies of the System PbO-ZnO-FeO-Fe2O3-CaO-SiO2-Al2O3 for Lead and Zinc Smelting. In: Zinc and Lead Processing; Dutrizac JE, Gonzalez JA, Bolton GL, Hancock P, Eds. Met. Soc. CIMM: 1998; p. 313-33.
Jak E, Hayes PC, Lee H-G. Improved methodologies for the determination of high temperature phase equilibria. Met Mater (Seoul). 1995;1(1):1-8. doi:10.1007/BF03055319
Jak E, Liu N, Hayes PC. Experimental study of Phase Equilibria in the Systems PbOx-CaO and PbOx-CaO-SiO2. Metall Mater Trans B. 1998;29B(3):541-53. doi:10.1007/s11663-998-0088-9
Jak E, Zhao B, Liu N, Hayes PC. Experimental Study of Phase Equilibria in the System PbO-ZnO-SiO2. Metall Mater Trans B. 1999;30B(1):21-7. doi:10.1007/s11663-999-0003-z
Decterov SA, Jak E, Hayes PC, Pelton AD. Experimental Study and Thermodynamic Optimization of the Fe-Zn-O System. Metall Mater Trans B. 2001;32(4):643-57. doi:10.1007/s11663-001-0119-2
Jak E, Decterov SA, Wu P, Hayes PC, Pelton AD. Thermodynamic Optimisation of the Systems PbO-SiO2, PbO-ZnO, ZnO-SiO2 and PbO-ZnO-SiO2. Metall Mater Trans B. 1997;28B:1011-8. doi:10.1007/s11663-997-0055-x
Jak E, Decterov SA, Hayes PC, Pelton AD. Thermodynamic Optimisation of the Systems CaO-Pb-O and PbO-CaO-SiO2. Can Metall Q. 1998;37(1):41-7. doi:10.1016/S0008-4433(98)00004-4
Jak E, Decterov S, Pelton AD, Hayes PC. Coupled Experimental and Thermodynamic Study of the Zn-Fe-Si-O System. Metall Mater Trans B. 2001;32B(5):793-800. doi:10.1007/s11663-001-0066-y
Jak E, Decterov SA, Hayes PC, Pelton AD. Thermodynamic Modelling of the System PbO-ZnO-FeO-Fe2O3-CaO-SiO2 for Zinc/Lead Smelting. In: Proc 5th Int Conf on Molten Slags, Fluxes and Salts; Iron and Steel Soc., AIME, Sydney, Australia: 1997; p. 621-8.
Decterov SA, Pelton AD. Thermodynamic Modeling of Lead Distribution among Matte, Slag and Liquid Copper. Metall Mater Trans B. 1999;30B(6):1033-44. doi:10.1007/s11663-999-0109-3
Decterov SA, Dessureault Y, Pelton AD. Thermodynamic Modeling of Zinc Distribution among Matte, Slag and Liquid Copper. Can Metall Q. 2000;39(1):43-54. doi:10.1179/cmq.2000.39.1.43
Prostakova V, Chen J, Jak E, Decterov SA. Experimental study and thermodynamic optimization of the CaO-NiO, MgO-NiO and NiO-SiO2 systems. CALPHAD. 2012;37:1-10. doi:10.1016/j.calphad.2011.12.009
Prostakova V, Chen J, Jak E, Decterov S. Experimental study and thermodynamic optimization of the MgO-NiO-SiO2 system. J Chem Thermodyn. 2013;62:43-55. doi:10.1016/j.jct.2013.02.019
Prostakova V. Development of a thermodynamic database for nickel containing oxide systems for simulation of nickel extraction from laterite ores [PhD thesis]. Montreal (Canada): École Polytechnique de Montréal; 2013. 320 p.
Prostakova V, Chen J, Jak E, Decterov SA. Experimental investigation and thermodynamic modeling of the (NiO + CaO + SiO2), (NiO + CaO + MgO) and (NiO + CaO + MgO + SiO2) systems. J Chem Thermodyn. 2015;86:130-42. doi:10.1016/j.jct.2015.01.017
Jung I-H, Decterov SA, Pelton AD. Thermodynamic modeling of the CoO-SiO2 and CoO-FeO-Fe2O3-SiO2 systems. Int J Mater Res. 2007;98(9):816-25. doi:10.3139/146.101538
Jung I-H, Decterov SA, Kim H-M, Kang Y-B, Pelton AD. Thermodynamic Evaluation and Modeling of the Fe-Co-O System. Acta Mater. 2004;52(2):507-19. doi:10.1016/j.actamat.2003.09.034
Jung I-H, Decterov SA, Pelton AD. Physico-chemical modeling of slags and mattes for Co and Ni production. In: Pyrometallurgy of Nickel and Cobalt 2009, Proc 48th Annual Conf of Metallurgists of CIM; Liu J, Peacey J, Barati M, Kashani-Nejad S, Davis B, Eds. CIM, Sudbury, Ontario, Canada, 2009; p. 317-29.
Decterov S, Pelton AD. Critical Evaluation and Optimization of the Thermodynamic Properties and Phase Diagrams of the CrO-Cr2O3, CrO-Cr2O3-Al2O3 and CrO-Cr2O3-CaO Systems. J Phase Equilib. 1996;17(6):476-87. doi:10.1007/BF02665994
Decterov SA, Pelton AD. Critical Evaluation and Optimization of the Thermodynamic Properties and Phase Diagrams of the CrO-Cr2O3-SiO2, and CrO-Cr2O3-SiO2-Al2O3 Systems. J Phase Equilib. 1996;17(6):488-94. doi:10.1007/BF02665995
Decterov SA, Pelton AD. Critical Evaluation and Optimization of the Thermodynamic Properties and Phase Diagram of the CrO-Cr2O3-SiO2-CaO System. Metall Mater Trans B. 1997;28B(2):235-42. doi:10.1007/s11663-997-0089-0
Jung I-H, Decterov SA, Pelton AD. Thermodynamic Modeling of the MgO-Al2O3-CrO-Cr2O3 System. J Am Ceram Soc. 2005;88(7):1921-8. doi:10.1111/j.1551-2916.2005.00336.x
Jung I-H, Kang Y-B, Decterov SA, Pelton AD. Thermodynamic Evaluation and Optimization of the MnO-Al2O3 and MnO-Al2O3-SiO2 Systems and Applications to Inclusion Engineering. Metall Mater Trans B. 2004;35(2):259-68. doi:10.1007/s11663-004-0027-3
Kang Y-B, Jung I-H, Decterov SA, Pelton AD, Lee H-G. Critical Thermodynamic Evaluation and Optimization of the CaO-MnO-SiO2 and CaO-MnO-Al2O3 Systems. ISIJ Int. 2004;44(6):965-74.
Kang Y-B, Jung I-H, Decterov SA, Pelton AD, Lee H-G. Phase Equilibria and Thermodynamic Properties of the CaO-MnO-Al2O3-SiO2 System by Critical Evaluation, Modeling and Experiment. ISIJ Int. 2004;44(6):975-83. doi:10.2355/isijinternational.44.975
Jak E, Hayes P, Pelton AD, Decterov SA. Thermodynamic modeling of the Al2O3–CaO–FeO–Fe2O3–PbO–SiO2–ZnO system with addition of K and Na with metallurgical applications. In: Proc VIII Int’l Conf on Molten Slags, Fluxes and Salts; Santiago, Chile, 2009; p. 473-90.
Decterov SA, Swamy V, Jung I-H. Thermodynamic modeling of the B2O3-SiO2 and B2O3-Al2O3 systems. Int J Mater Res. 2007;98(10):987-94. doi:10.3139/146.101555
Swamy V, Jung I-H, Decterov SA. Thermodynamic modeling of the Al2O3-B2O3-SiO2 system. J Non-Cryst Solids. 2009;355(34-36):1679-86. doi:10.1016/j.jnoncrysol.2009.06.036
Shukla A. Development of a critically evaluated thermodynamic database for the systems containing alkaline-earth oxides [PhD thesis]. Montreal (Canada): École Polytechnique de Montréal; 2012. 321 p.
Shukla A, Decterov SA, Pelton AD. Thermodynamic Evaluation and Optimization of the SrO-MgO, SrO-SiO2 and SrO-MgO-SiO2 Systems. J Phase Equilib Diffus. 2017;38(5):615-29. doi:10.1007/s11669-017-0585-0
Shukla A, Jung IH, Decterov SA, Pelton AD. Thermodynamic evaluation and optimization of the BaO-SiO2 and BaO-CaO-SiO2 systems. CALPHAD. 2018;61:140-7. doi:10.1016/j.calphad.2018.03.001
Shishin D, Jak E, Decterov SA. Critical Assessment and Thermodynamic Modeling of the Fe-O-S System. J Phase Equilib Diffus. 2015;36(3):224-40. doi:10.1007/s11669-015-0376-4
Lee BJ. Revision of thermodynamic descriptions of the Fe-Cr and Fe-Ni liquid phases. CALPHAD. 1993;17(3):251-68. doi:10.1016/0364-5916(93)90004-U
Ansara I, Dupin N, Leo LH, Sundman B. Thermodynamic assessment of the Al-Ni system. J Alloys Compd. 1997;247(1-2):20-30. doi:10.1016/s0925-8388(96)02652-7
Nekhoroshev E, Jak E, Decterov SA. Thermodynamic modeling of the Na2O-SiO2 system, in preparation.
Nekhoroshev E, Decterov SA. Thermodynamic modeling of the Na2O-B2O3 system, in preparation.
Pelton AD, Decterov SA, Eriksson G, Robelin C, Dessureault Y. The Modified Quasichemical Model. I - Binary Solutions. Metall Mater Trans B. 2000;31(4):651-9. doi:10.1007/s11663-000-0103-2
Pelton AD, Chartrand P. The Modified Quasichemical Model. II - Multicomponent Solutions. Metall Mater Trans A. 2001;32(6):1355-60. doi:10.1007/s11661-001-0226-3
Dingwell DB, Pichavant M, Holtz F. Experimental studies of boron in granitic melts. In: Reviews in Mineralogy. 33; Grew ES, Anovitz LM, editors. Washington, D.C.: Mineralogical Society of America; 1996. p. 331-85.
Brosh E, Pelton AD, Decterov SA. A Model to Calculate the Viscosity of Silicate Melts. Part V: Borosilicate melts containing alkali oxides. Int J Mat Res. 2012;103(5):537-50. doi:10.3139/146.110639
Shelby JE. Introduction to Glass Science and Technology, 2d Edition: The Royal Society of Chemistry, Thomas Graham House, Science Park, Milton Road, Cambridge CB4 OWF, UK; 2005. 291 p.
Uhlmann DR, Shaw RR. Thermal expansion of alkali borate glasses and the boric oxide anomaly. J Non-Cryst Solids. 1969;1(5):347-59. doi:10.1016/0022-3093(69)90018-0
Kang Y-B, Pelton A. Thermodynamic Model and Database for Sulfides Dissolved in Molten Oxide Slags. Metall Mater Trans B. 2009;40:979-94. doi:10.1007/s11663-009-9283-6
Pelton AD. Thermodynamic Calculations of Chemical Solubilities of Gases in Oxide Melts and Glasses. Glastech Ber. 1999;72(7):214-26.
Hillert M, Jansson B, Sundman B. Application of the Compound-Energy Model to Oxide Systems. Z Metallkd. 1988;79(2):81-7.
Hillert M, Staffansson L-I. The Regular Solution Model for Stoichiometric Phases and Ionic Melts. Acta Chem Scand. 1970;24:3618-26. doi:10.3891/acta.chem.scand.24-3618
Decterov SA, Pelton AD, Seifert H-J, Fabrichnaya O, Hajra JP, Navrotsky A, et al. Thermodynamic Modelling of Oxide and Oxynitride Phases. Z Metallkd. 2001;92:533-49.
Hillert M, Kjellqvist L, Mao H, Selleby M, Sundman B. Parameters in the compound energy formalism for ionic systems. CALPHAD. 2009;33(1):227-32. doi:10.1016/j.calphad.2008.05.006
Carpenter MA. Kinetic Control of Ordering and Exsolution in Omphacites. Contrib Mineral Petrol. 1978;67(1):17-24. doi:10.1007/BF00371629
Davidson PM, Burton BP. Order-Disorder in Omphacitic Pyroxenes: A Model for Coupled Substitition in the Point Approximation. Am Mineral. 1987;72(3-4):337-44.
Cohen R. Thermodynamic solution properties of aluminous clinopyroxenes: Nonlinear least squares refinements. Geochim Cosmochim Acta. 1986;50:563-75. doi:10.1016/0016-7037(86)90105-5
Kurepin VA. Thermodynamic model of a heterovalent solid solution at local electroneutrality. Geokhimiya. 1978;1:16-33.
Kerrick DM, Darken LS. Statistical thermodynamic models for ideal oxide and silicate solid solutions, with application to plagioclase. Geochim Cosmochim Acta. 1975;39(10):1431-42. doi:10.1016/0016-7037(75)90122-2
Chartrand P, Pelton AD. The Modified Quasichemical Model. III - Two Sublattices. Metall Mater Trans A. 2001;32(6):1397-407. doi:10.1007/s11661-001-0229-0
Pelton AD, Chartrand P, Eriksson G. The modified Quasichemical Model. IV - Two Sublattice Quadruplet Approximation. Metall Mater Trans A. 2001;32(6):1409-15. doi:10.1007/s11661-001-0230-7
Navrotsky A. Repeating Patterns in Mineral Energetics. Am Mineral. 1994;79(7-8):589-605.
Carpenter MA, Putnis A, Navrotsky A, McConnell JDD. Enthalpy Effects Associated with Al/Si Ordering in Anhydrous Mg-Cordierite. Geochim Cosmochim Acta. 1983;47(5):899-906. doi:10.1016/0016-7037(83)90155-2
Newton RC, Wood BJ, Kleppa OJ. Thermochemistry of Silicate Solid Solutions. Bull Mineral. 1981;104(2-3):162-71.
Navrotsky A. Crystal Chemical Constraints on the Thermochemistry of Minerals. In: Reviews in Mineralogy. 14 (Microscopic to Macroscopic); Kieffer SW, Navrotsky A, editors. Washington, D. C.: Mineralogical Society of America; 1985. p. 225-75.
Wriedt HA. The Fe-O (Iron-Oxygen) System. J Phase Equilib. 1991;12(2):170-200. doi:10.1007/bf02645713
Vallet P, Carel C. The Fe-O (iron-oxygen) phase diagram in the range of the nonstoichiometric monoxide and magnetite at the Fe-rich limit: reduction diagrams. Bull Alloy Phase Diagrams. 1989;10(3):209-18. doi:10.1007/bf02877494
Hidayat T, Shishin D, Jak E, Decterov S. Thermodynamic Reevaluation of the Fe-O System. CALPHAD. 2015;48:131-44. doi:10.1016/j.calphad.2014.12.005
Todd SS, Bonnickson KR. Low Temperature Heat Capacities and Entropies at 298.16°K of Ferrous Oxide, Manganous Oxide and Vanadium Monoxide. J Am Chem Soc. 1951;73(8):3894-5. doi:10.1021/ja01152a099
Gronvold F, Stolen S, Tolmach P, Westrum EFJ. Heat capacities of the wustites Fe0.9379O and Fe0.9254O at temperatures T from 5 K to 350 K. Thermodynamics of the reaction: xFe(s)+(1/4)Fe3O4(s)=Fe0.7500+xO(s)=Fe1-yO(s) at T=850 K, and properties of Fe1-yO(s) to T=1000 K. Thermodynamics of formation of wustite. J Chem Thermodyn. 1993;25(9):1089-117. doi:10.1006/jcht.1993.1107
Stolen S, Glockner R, Gronvold F, Atake T, Izumisawa S. Heat capacity and thermodynamic properties of nearly stoichiometric wustite from 13 to 450 K. Am Mineral. 1996;81(7-8):973-81.
Pelton AD. A General "Geometric" Thermodynamic Model for Multicomponent Solutions. CALPHAD. 2001;25(2):319-28. doi:10.1016/S0364-5916(01)00052-9
Grundy AN, Jung I-H, Pelton AD, Decterov SA. A Model to Calculate the Viscosity of Silicate Melts. Part II.: Viscosity of the Multicomponent NaO0.5-MgO-CaO-AlO1.5-SiO2 System. Int J Mat Res. 2008;99(11):1195-209. doi:10.3139/146.101753
Grundy AN, Liu H-C, Jung I-H, Decterov SA, Pelton AD. A Model to Calculate the Viscosity of Silicate Melts. Part I.: Viscosity of Binary SiO2-MeOx Systems (Me = Na, K, Ca, Mg, Al). Int J Mat Res. 2008;99(11):1185-94. doi:10.3139/146.101752
Kim W-Y, Yang X, Yan L, Pelton AD, Decterov SA. Modeling Viscosity of Silicate Melts Containing Zinc Oxide. CALPHAD. 2011;35(4):542-50. doi:10.1016/j.calphad.2011.09.005
Brosh E, Pelton AD, Decterov SA. A Model to Calculate the Viscosity of Silicate Melts. Part IV: Borosilicate melts. Int J Mat Res. 2012;103(4):494-501. doi:10.3139/146.110638
Kim W-Y, Pelton AD, Decterov SA. A Model to Calculate the Viscosity of Silicate Melts. Part III: Modification of the model for melts containing alkali metals. Int J Mat Res. 2012;103(3):313-28. doi:10.3139/146.110637
Kim W-Y, Pelton AD, Decterov SA. Modeling the Viscosity of Silicate Melts Containing Lead Oxide. Metall Mater Trans B. 2012;43(2):325-36. doi:10.1007/s11663-011-9610-6
Kim W-Y, Bale CW, Bélisle E, Pelton AD, Decterov SA. Modeling the viscosity of silicate melts containing manganese oxide. J Min Metall, Sect B. 2013;49(3):323-37. doi:10.2298/JMMB120918039K
Decterov SA, Kim W-Y, Pelton AD. A model and database for the viscosity of oxide glasses and melts. In: Proc 10th International Conference on Molten Slags, Fluxes and Salts (Molten16); Seattle, Washington, USA, 2016.
Preston E. The viscosity of the soda-silica glasses at high temperatures and its bearing on their constitution. J Soc Glass Technol. 1938;22:45-82.
Bockris JOM, Mackenzie JD, A. KJ. Viscous Flow in Silica and Binary Liquid Silicates. Trans Faraday Soc. 1955;51(12):1734-48. doi:10.1039/TF9555101734
Nemilov SV. Viscosity of glasses of sodium oxide-potassium oxide-silicon dioxide and lithium oxide-potassium oxide-silicon dioxide systems in softening point regions. Zhurnal Prikladnoi Khimii (Sankt-Peterburg, Russian Federation). 1969;42(1):55-62.
Sasek L, Meissnerova H, Prochazka J. Structure and properties of silicate melts. 7. Effect of the size of the Me+ and Me++ ions on the viscosity of silicate glass melts. Sbornik Vysoke Skoly Chemicko-Technologicke v Praze, L: Chemie a Technologie Silikatu. 1975;L6:95-129.
Knoche R, Dingwell DB, Seifert FA, Webb SL. Non-linear Properties of Supercooled Liquids in the System Na2O-SiO2. Chem Geol. 1994;116(1-2):1-16. doi:10.1016/0009-2541(94)90154-6
Ota R, Wakasugi T, Kawamura W, Tuchiya B, Fukunaga J. Glass Formation and Crystallization in Li2O-Na2O-K2O-SiO2. J Non-Cryst Solids. 1995;188(1-2):136-46. doi:10.1016/0022-3093(95)00185-9
Ehrt D, Keding R. Electrical conductivity and viscosity of borosilicate glasses and melts. Phys Chem Glasses: Eur J Glass Sci Technol, Part B. 2009;50(3):165-71.
Shartsis L, Spinner S, Capps W. Density, Expansivity, and Viscosity of Molten Alkali Silicates. J Am Ceram Soc. 1952;35:155-60. doi:10.1111/j.1151-2916.1952.tb13090.x
Eipeltauer E, More A. Viscosity of Binary Potassium Silicate Glasses. Radex Rundsch. 1960;4:230-8.
Pohlmann HJ. Investigation of viscosity of glasses in the silica-rich part of the system potassium oxide-lead(II) oxide-silicon dioxide. Glastech Ber. 1976;49(8):177-82.
Mizoguchi K, Okamoto K, Suginohara Y. Oxygen Coordination of Al3+ Ion in Several Silicate Melts Studied by Viscosity Measurements. Nippon Kinzoku Gakkaishi. 1982;46(11):1055-60.
Asayama E, Takebe H, Morinaga K. Critical Cooling Rates for the Formation of Glass for Silicate Melts. ISIJ Int. 1993;33(1):233-8. doi:10.2355/isijinternational.33.233
Kim K-D, Lee S-H. Viscosity behavior and mixed alkali effect of alkali aluminosilicate glass melts. J Ceram Soc Jpn. 1997;105(Oct.):827-32. doi:10.2109/jcersj.105.827
Riebling EF. Structure of Sodium Aluminosilicate Melts Containing at least 50 Mole % SiO2 at 1500°C. J Chem Phys. 1966;44(8):2857-65. doi:10.1063/1.1727145
Neuville DR, Richet P. Viscosity and entropy of molten silicates. Rivista della Stazione Sperimentale del Vetro (Murano, Italy). 1990;20(6):213-20.
Toplis MJ, Dingwell DB, Lenci T. Peraluminous Viscosity Maxima in Na2O-Al2O3-SiO2 Liquids: the Role of Triclusters in Tectosilicate Melts. Geochim Cosmochim Acta. 1997;61(13):2605-12. doi:10.1016/S0016-7037(97)00126-9
Oshchipkov FP, Rabinovich BV. Viscosity of nonsilicate glasses. Akad Nauk SSSR, Otdel Tekh Nauk, Inst Mashinovedeniya, Soveshchanie Vyazkosti Zhidkostei i Kolloid Rastvorov [Conference on Viscosity of Liquids and Colloidal Solutions]. 1941;1:353-7. Russian.
Shartsis L, Capps W, Spinner S. Viscosity and Electrical Resistivity of Molten Alkali Borates. J Am Ceram Soc. 1953;36(10):319-26. doi:10.1111/j.1151-2916.1953.tb12808.x
Nemilov SV. Studies on the structure of glasses in B2O3-Na2O system by the viscosimetric method. Izvestiya Akademii Nauk SSSR, Neorganicheskie Materialy. 1966;2(2):349-56. Russian.
Frumin EI, Yakobashvili SB. Surface tension, density, and viscosity of molten borates. In: Fiz Khim Poverkh Yavlenii Vys Temp; Eremenko VN, Ed. "Naukova Dumka", Kiev, USSR: 1971; p. 116-20. Russian.
Visser TJM, Stevels JM. Rheological properties of boric oxide and alkali borate glasses. J Non-Cryst Solids. 1972;7(4):376-94. doi:10.1016/0022-3093(72)90272-4
Kaiura GH, Toguri JM. The viscosity and structure of sodium borate melts. Phys Chem Glasses. 1976;17(3):62-9.
Leedecke CJ, Bergeron CG. The growth of potassium borate (K2B8O13) in its stoichiometric melt. J Cryst Growth. 1976;32(3):327-31. doi:10.1016/0022-0248(76)90113-5
Sakka S, Kamiya K, Matsushita K, Okamura T. Viscosity of mixed-alkali borate glasses. Res Rep Fac Eng Mie Univ. 1976;1:47-58.
Sasek L, Kovandova J, Drahonovsky M. Viscosity of soda-borate glasses and glass melts. Sbornik Vysoke Skoly Chemicko-Technologicke v Praze, L: Chemie a Technologie Silikatu. 1984;L12:47-72.
Yamate T, Kadogawa Y. Effect of glass composition on its viscosity. Viscosity of binary alkali borate glasses. Kenkyu Hokoku - Asahi Garasu Kogyo Gijutsu Shoreikai. 1984;44:15-24.
Startsev YK, Safutina TV. Influence of residual water in a glass on its rheological and relaxation properties (by the example of a 5Na2O·95B2O3 glass). Glass Phys Chem. 2001;27(5):411-7. doi:10.1023/A:1012443530817
Jung I-H, Decterov SA, Pelton AD. Computer Applications of Thermodynamic Databases to Inclusion Engineering. ISIJ Int. 2004;44(3):527-36. doi:10.2355/isijinternational.44.527
Decterov SA, Kang Y-B, Jung I-H. Thermodynamic Database for the Al-Ca-Co-Cr-Fe-Mg-Mn-Ni-Si-O-P-S System and Applications in Ferrous Process Metallurgy. J Phase Equilib Diffus. 2009;30(5):443-61. doi:10.1007/s11669-009-9569-z
Decterov SA, Pelton AD. Thermodynamic Calculation of Gas/Slag/Refractory Equilibria in Coal Gasification. In: Ceramic Transactions, vol 78: Corrosion of Materials by Molten Glass; Pecoraro G, Ed. Amer. Ceram. Soc.: 1996; p. 91-103.
Jung I-H, Decterov SA, Pelton AD. Computer Application of Thermodynamic Database to Corrosion of Refractories. In: Proc UNITECR 2003 Congress (Oct 2003, Osaka, Japan); 2003; p. 252-5.
Jung I-H, Decterov SA, Pelton AD. Computer application of thermodynamic database to corrosion of refractories. Taikabutsu. 2004;56(8):382-6.
Jak E, Hayes P, Bale CW, Decterov SA. Application of FactSage thermodynamic modeling of recycled slags (Al2O3-CaO-FeO-Fe2O3-SiO2-PbO-ZnO) in the treatment of wastes from end-of-life-vehicles. Int J Mater Res. 2007;98(9):872-8. doi:10.3139/146.101546
Hidayat T, Jak E. A thermodynamic optimization of “Cu2O”-containing slags systems. In: Ninth International Conference on Molten Slags, Fluxes and Salts (MOLTEN12); The Chinese Society for Metals Beijing, China, 2012.
Hack K. SGTE Casebook: Thermodynamics at Work, Second Edition. Cambridge, England: CRC Press, Boca Raton Boston New York Washington, DC; 2008. 453 p.
Pelton AD. Thermodynamics and Phase Diagrams. In: Physical Metallurgy; Laughlin DE, Hono K, editors. Elsevier; 2014. p. 203-303.
Gheribi AE, Audet C, Le Digabel S, Belisle E, Bale CW, Pelton AD. Calculating optimal conditions for alloy and process design using thermodynamic and property databases, the FactSage software and the Mesh Adaptive Direct Search algorithm. CALPHAD. 2012;36:135-43. doi:10.1016/j.calphad.2011.06.003
Gheribi AE, Harvey J-P, Belisle E, Robelin C, Chartrand P, Pelton AD, et al. Use of a biobjective direct search algorithm in the process design of material science applications. Optim Eng. 2016;17:27–45. doi:10.1007/s11081-015-9301-2
Audet C, Le Digabel S. The mesh adaptive direct search algorithm for periodic variables (PJO). Pacific Journal of Optimization. 2012;8(1):103-19.
Petersen S, Hack K. The thermochemistry library ChemApp and its applications. Int J Mater Res. 2007;98(10):935-45. doi:10.3139/146.101551
DOI: https://doi.org/10.15826/chimtech.2018.5.1.02
Copyright (c) 2017 Sergei Alexander Decterov

This work is licensed under a Creative Commons Attribution 4.0 International License.
Chimica Techno Acta, 2014–2025
eISSN 2411-1414
Copyright Notice






