Phase equilibria, crystal structure and oxygen nonstoichiometry of the complex oxides in the Sm – (Sr, Ba) – (Co, Fe) – O systems
Abstract
Keywords
Full Text:
PDFReferences
Tu HY, Takeda Y, Imanishi N, Yamamoto O. Ln1-xSrxCoO3 (Ln = Sm, Dy) for the electrode of solid oxide fuel cells. Solid State Ionics. 1997;100:283–8. doi:10.1016/S0167-2738(97)00360-3
Kim J-H, Manthiram A. LnBaCo2O5+d oxides as cathodes for intermediate-temperature solid oxide fuel cells. J Electrochem Soc. 2008;155:B385–90. doi:10.1149/1.2839028
Zhou Q, He T, Ji Y. SmBaCo2O5+x double-perovskite structure cathode material for intermediate-temperature solid-oxide fuel cells. J Power Sources. 2008;185:754–8. doi:10.1016/j.jpowsour.2008.07.064
Chen D, Wang F, Shi H, Ran R, Shao Z. Systematic evaluation of Co-free LnBaFe2O5+ı (Ln = Lanthanides or Y) oxides towards the application as cathodes for intermediate-temperature solid oxide fuel cells. Electrochimica Acta. 2012;78:466–74. doi:10.1016/j.electacta.2012.06.073
Kim J-H, Manthiram A. Characterization of Sr2.7Ln0.3Fe1.4Co0.6O7 (Ln=La,Nd,Sm,Gd) intergrowth oxides as cathodes for solid oxide fuel cells. Solid State Ionics. 2009;180:1478–83. doi:10.1016/j.ssi.2009.09.007
Chavez E, Mueller M, Mogni L, Caneiro A. Study of LnBaCo2O6-d (Ln=Pr, Nd, Sm and Gd) double perovskite as new cathode material for IT-SOFC. J Phys: Conf Ser. 2009;167(012043):1–6. doi:10.1088/1742-6596/167/1/012043
Li Q, Zhu X, Yang W. Single-step fabrication of asymmetric dual-phase composite membranes for oxygen separation. J Membr Sci. 2008;325:11–5. doi:10.1016/j.memsci.2008.08.002
Kovalevsky AV, Kharton VV, Tikhonovich VN, Naumovich EN, Tonoyan AA, Reut OP, Boginsky LS. Oxygen permeation through Sr(Ln)CoO3- (Ln=La, Nd, Sm, Gd) ceramic membranes. Mater Sci Eng. 1998;B52:105–16. doi:10.1016/S0921-5107(97)00292-4
Ikeguchi M, Mimura T, Sekine Y, Kikuchi E, Matsukata M. Reaction and oxygen permeation studies in Sm0.4Ba0.6Fe0.8Co0.2O3-d membrane reactor for partial oxidation of methane to syngas. Appl Catal, A. 2005;290:212–20. doi:10.1016/j.apcata.2005.05.033
Lechevalliera L, Le Breton JM, Wang JF, Harris IR. Structural analysis of hydrothermally synthesized Sr1-xSmxFe12O19 hexagonal ferrites. J Magn Magn Mater. 2004;269:192–6. doi:10.1016/S0304-8853(03)00591-2
Wang JF, Ponton CB, Harris IR. A study of Sm-substituted SrM magnets sintered using hydrothermally synthesized powders. J Magn Magn Mater. 2006;298:122–31. doi:10.1016/j.jmmm.2005.03.012
Lechevallier L, Le Breton JM, Morel A, Teillet J. Structural and magnetic properties of Sr1-xSmxFe12O19 hexagonal ferrites synthesised by a ceramic process. J Alloys Compd. 2003;359:310–4. doi:10.1016/S0925-8388(03)00206-8
Arakawa T, Tsuchi-ya S-ishi, Shiokawa J. Catalytic properties and activity of rare earth orthoferrites in oxidation of methanol. J Catal. 1982;74:317–22. doi:10.1016/0021-9517(82)90037-9
Li L, Wang X, Zhang Y. Enhanced visible light-responsive photocatalytic activity of LnFeO3 (Ln = La, Sm) nanoparticles by synergistic catalysts. Mater Res Bull. 2014;50:18–22. doi:10.1016/j.materresbull.2013.10.027
Sazonov LA, Moskvina ZV, Artamonov EV. Investigation of catalytic properties of compounds LnMeO3 type in the reactions of homomolecular exchange of oxygen. Kinet Catal. 1974;15(1):120–6.
Arakawa T, Yoshida A, Shiokawa J. Catalytic properties of rare earth cobaltites and related compounds. Mater Res Bull. 1980;15(3):347–52. doi:10.1016/0025-5408(80)90178-6
Michel CR, Delgado E, Santillán G, Martínez AH, Chávez-Chávez A. An alternative gas sensor material: Synthesis and electrical characterization of SmCoO3. Mater Res Bull. 2007;42:84–93. doi:10.1016/j.materresbull.2006.05.008
Delgado E, Michel CR. CO2 and O2 sensing behavior of nanostructured barium - doped SmCoO3. Mater Lett. 2006:60;1613–6. doi:10.1016/j.matlet.2005.11.080
Mochinaga R, Yamasaki T, Arakawa T. The gas-sensing of SmCoOX/MOX (M=Fe, Zn, In, Sn) having a heterojunction. Sens Actuators, B: Chemical. 1998;52(1–2):96–9. doi:10.1016/S0925-4005(98)00262-7
Kropanev AYu, Petrov AN, Zhukovsky VM. The phase diagrams of the Ln – Co – O systems (Ln = Sm, Eu, Gd, Tb, Dy, Ho). Russ J Inorg Chem. 1983;28(11):2938–43.
Kropanev AYu, Petrov AN. Thermal stability of SmCoO3, EuCoO3, GdCoO3, TbCoO3, DyCoO3, HoCoO3 cobaltites in air. Inorg Mater. 1983;19(12):1782–5.
Kitayama K. Thermogravimetric study of the Ln2O3-Co-Co2O3 system III. Ln=Pr, Sm, Eu and Tb. J Solid State Chem. 1988;77(2): 366–75. doi:10.1016/0022-4596(88)90260-5
Wold A, Ward R. Perovskite type oxides of cobalt, chromium and vanadium with some rare earth elements. J Am Chem Soc. 1954;76(4):1029–30. doi:10.1021/ja01633a031
Bertaut F, Forrat F. Sur les deformations dans les perovskites a base de terres rares et d’elements de transition trivalents. J Phys Radium. 1956;17:129–31. doi:10.1051/jphysrad:01956001702012900
Kappatsch A, Qezel-Ambrunaz S, Sivardiere J. Structure et properties magnetiques des orthocobaltites de terres rares TCoO3. J Phys France. 1970;31(4):369–76. doi:10.1051/jphys:01970003104036900
Demazeau G, Pouchard M, Hagenmuller P. Sur de nouveaux composes oxygenes du cobalt +III derives de la perovskite. J Solid State Chem. 1974;9(3):202–9. doi:10.1016/0022-4596(74)90075-9
Demazeau G, Pouchard M, Hagenmuller P. Les composes oxygenes ternaries du cobalt +3 et des terres. C r Acad Sci. 1973;277(2):109–12.
Pérez-Cacho J, Blasco J, García J, Sanchez R. Relationships between Structure and Physical Properties in SmNi1-xCoxO3. J Solid State Chem. 2000;150:145–53. doi:10.1006/jssc.1999.8570
Petrov AN, Kropanev AYu, Zhukovsky VM, Cherepanov VA, Neudachina GK. The conditions and mechanism of solid state synthesis of the rare earth cobaltates RCoO3 (R=La, Pr, Nd, Sm, Gd). Zhurnal Neorganicheskoi Khimii. 1981;26(12):3190–4.
Kropanev AYu, Petrov AN, Rabinovich LYa. Investigations of solid state interactions of Ln2O3 with CoO (Ln = Sm, Eu, Gd, Dy, Ho). Zhurnal Neorganicheskoi Khimii. 1983;28(10):2609–12.
Kropanev AYu, Petrov AN, Rabinovich LYa. Solid state synthesis of RE cobaltites with RCoO3 composition (R – Sm, Eu. Gd). Inorg Mater 1984;20(1):116-20.
Kniga MV, Akhrem LI. Kinetics of reaction of samarium oxide and cobalt oxide. Izv AN BSSR Ser Khim Nauk. 1968;2:56–60.
Petrov AN, Kropanev AYu, Zhukovsky VM. Thermodynamic properties of rare earth cobaltites with composition RCoO3. Zhurnal Fizicheskoj Khimii. 1984;58(1):50–3.
Sabasri R, Pankajavalli R, Sreedharan OM. High temperature thermodynamic stabilities of RCoO3 (R=Nd, Sm,Eu, Gd or Dy) using solid oxide-electrolyte emf technique. J Alloy Compd. 1998;269:71–4. doi:10.1016/S0925-8388(98)00004-8
Kitayama K, Katsura T. Phase equilibria in Fe-Fe2O3-Ln2O3 (Ln=Sm and Er) systems at 1200°C. Bull Chem Soc Jpn. 1976;49(4):998–1001. doi:10.1246/bcsj.49.998
Parida SC, Jacob KT, Venugopal V. Thermodynamic Properties of SmFeO3(s) and Sm3Fe5O12 (s). J Phase Equilibria. 2003;24(5):431-40. doi:10.1361/1054971037
Geller S, Wood EA. Crystallographic studies of perovstite-like compounds. I. Rare earth orthferrites and YFeO3, YCrO3, YAlO3. Acta Crystallogr. 1956;9:563–8. doi:10.1107/S0365110X56001571
Marezio M, Remeika JP, Dernier PD. The crystal chemistry of the rare earth orthoferrites. Acta Crystallogr. 1970;B26:2008–22. doi:10.1107/S0567740870005319
Berenov A, Angeles E, Rossiny J, Raj E, Kilner J, Atkinson A. Structure and transport in rare-earth ferrates. Solid State Ionics. 2008;179:1090–3. doi:10.1016/j.ssi.2008.01.025
Porta P, Cimino S, De Rossi S, Faticanti M, Minelli G, Pettiti I. AFeO3 (A=La, Nd, Sm) and LaFe1−xMgxO3 perovskites: structural and redox properties. Mater Chem Phys. 2001;71:165–73. doi:10.1016/S0254-0584(01)00273-5
Niu X, Li H, Liu G. Preparation, characterization and photocatalytic properties of REFeO3 (RE = Sm, Eu, Gd). J Mol Catal A: Chem. 2005;232:89–93. doi:10.1016/j.molcata.2005.01.022
Espinosa GP. Crystal chemistry study of the rare-earth iron garnets. J Chem Phys. 1962;10:2344–7. doi:10.1063/1.1733008
Narayanan VKS, Gajbhiye NS, Bahadur D. Characterization of dysprosium and samarium iron garnets synthesized by the citrate gel process. J Mater Sci Lett. 1987;6:281–4. doi:10.1007/BF01729325
Guillot M, Rodic D, Mitric M. Temperature dependencies of the lattice constants and thermal expansion coefficients of Sm3Fe5O12 and Er3Fe5O12 single crystals. J Appl Phys. 993;73(1):6304–6. doi:10.1063/1.352678
Cheng Z, Yang H. Synthesis and magnetic properties of Sm–Y3Fe5O12 nanoparticles. Physica E. 2007;39:198–202. doi:10.1016/j.physe.2007.04.003
Kniga MV, Rubinchik YaS, Kuligina MP. Kinetics of reaction in the Sm2O3-Fe2O3 system. Izv AN BSSR Ser Khim Nauk. 1969;3:30–3.
Katsura T, Kitayama K, Sugihara T, Kimizuka N. Thermochemical properties of Lanthanoid-Iron-Perovskite at high temperatures. Bull Chem Soc Jpn. 1975;48(6):1809–11.
Katsura T, Sekine T, Kitayama K, Sugihara T. Thermodynamic properties of Fe-Lanthanoid-O compounds at high temperatures. J Solid State Chem. 1978;23:43–57. doi:10.1016/0022-4596(78)90052-X
Kimizuka N, Yamamoto A, Ohashi H, Sugihara T, Sekine T. The stability of the phases in the Ln2O3-FeO-Fe2O3 systems which are stable at elevated temperatures (Ln: lanthanide elements and Y). J Solid State Chem. 1983;49:65–76. doi:10.1016/0022-4596(83)90217-7
Istomin SYa, Drozhzhin OA, Svensson G, Antipov EV. Synthesis and characterization of Sr1−xLnxCoO3−δ, Ln = Y, Sm–Tm, 0.1≤x≤0.5. Solid State Scien. 2004;6:539–46. doi:10.1016/j.solidstatesciences.2004.03.029
Kovalevsky AV, Kharton VV, Tikhonovich VN, Naumovich EN, Tonoyan AA, Reut OP, Boginsky LS. Oxygen permeation through Sr(Ln)CoO3-d (Ln=La, Nd, Sm, Gd) ceramic membranes. Mater Sci Eng B. 1998;B52:105–16. doi:10.1016/S0921-5107(97)00292-4
Jung KH, Choi S-M, Park H-H, Seo W-S. High temperature thermoelectric properties of Sr and Fe doped SmCoO3 perovskite structure. Current Applied Physics. 2011;11:S260–5. doi:10.1016/j.cap.2010.12.032
Kang JW, Ryu KH, Yo CH. Studies of nonstoichiometry and physical properties of the perovskite Sm1-xSrxCoO3-y system. Bull Korean Chem Soc. 1995;16(7):600–3.
James M, Cassidy D, Glossens DJ, Withers RL. The phase diagram and tetragonal superstructures of the rare earth cobaltate phases Ln1-xSrxCoO3-δ (Ln = La 3+, Pr 3+, Nd 3+, Sm 3+, Gd3+,Y3+, Ho 3+, Dy3+,
Er 3+, Tm 3+ and Yb3+). J Solid State Chem. 2004;177:1886–95. doi:10.1016/j.jssc.2004.01.012
James M, Cassidy D, Wilson KF, Horvat J, Withers RL. Oxygen vacancy ordering and magnetism in the rare earth stabilized perovskite form of “SrCoO3−δ”. Solid State Scien. 2004;6:655–62. doi:10.1016/j.solidstatesciences.2003.03.001
Baek SW, Kim JH, Bae J. Characteristics of ABO3 and A2BO4 (A = Sm, Sr; B = Co, Fe, Ni) samarium oxide system as cathode material for intermediate temperature-operating solid oxide fuel cell. Solid State Ionics. 2008;179:1570–4. doi:10.1016/j.ssi.2007.12.010
Yang S, He T, He Q. Sm0.5Sr0.5CoO3 cathode material from glycine-nitrate process: Formation, characterization, and application in LaGaO3-based solid oxide fuel cells. J Alloys Compd. 2008;450:400–4. doi:10.1016/j.jallcom.2006.10.147
Dong F, Chen D, Ran R, Park H, Kwak C, Shao Z. A comparative study of Sm0.5Sr0.5MO3-d (M = Co and Mn) as oxygen reduction electrodes for solid oxide fuel cells. Int J Hydrogen Energy. 2012; 37:4377–87. doi:10.1016/j.ijhydene.2011.11.150
Volkova NE, Maklakova AV, Gavrilova LYa, Cherepanov VA. Phase equilibria, crystal structure and properties of intermediate oxides in the Sm2O3 – SrO – CoO system. Eur J Inorg Chem. 2017;2017(26):3285–92. doi:10.1002/ejic.201700321
Cherepanov VA, Barkhatova LYu, Petrov AN, Voronin VI. Phase equilibria in the La-Sr-Co-O system and thermodynamic stability of the single phases. Solid Oxide Fuel Cells IV. M.Dokiya, O.Yamamoto, H.Tagawa, S.C.Singhal, editors. PV 95-1, p.434–443, The Electrochemical Society Proceedings Series, Pennington, NJ (1995).
Song HS, Min J-H, Kim J, Moon J. Phase stability of Sm0.5Sr0.5CoO3 cathodes for on-planar type, single-chamber, solid oxide fuel cells. J Power Sources. 2009;191:269–74. doi:10.1016/j.jpowsour.2009.02.028
Wang Y, Nie H, Wang S, Wen T-L, Guth U, Valshook V. A2-αA’αBO4-type oxides as cathode materials for IT-SOFCs (A = Pr, Sm; A’ = Sr; B = Fe, Co). Mater Lett. 2006;60:1174–8. doi:10.1016/j.matlet.2005.10.104
James M, Tedesco A, Cassidy D, Colella M, Smythe PJ. The phase diagram and crystal chemistry of strontium-doped rare earth cobaltates: Ln2−xSrxCoO4+δ (Ln = La–Dy). J Alloy Compd. 2006;419:201–7. doi:10.1016/j.jallcom.2005.08.080
Siwen L, Yufang R. The synthesis and physical properties of the new layered lanthanide alkaline earth cobalt oxides [Ln2MCo2O7 (Ln = Sm, Gd; M = Sr,Ba)]. Mater Res Bull. 1994;29:993–1000. doi:10.1016/0025-5408(94)90061-2
Park SK, Ishikawa T, Tokura Y, Li JQ, Matsui Y. Variation of charge-ordering in R1/3Sr2/3FeO3 (R=La, Pr, Nd, Sm, and Gd). Phys Rev B. 1999;60:10788–95. doi:10.1103/PhysRevB.60.10788
Zhao YM, Hervieu M, Nguyen N, Raveau B. Charge Ordering and Magnetotransport Transitions in Sm1/3Sr2/3FeO3-. J Solid State Chem. 2000;153:140–4. doi:10.1006/jssc.2000.8763
Lu X, Chen Y, Ding Y, Lin B. A cobalt-free Sm0.5Sr0.5FeO3-δ BaZr0.1Ce0.7Y0.2O3-δ composite cathode for proton-conducting solid oxide fuel cells. Int J Hydrogen Energy. 2012;37:8630–4. doi:10.1016/j.ijhydene.2012.02.050
Anderson MD, Stevenson JW, Simner SP. Reactivity of lanthanide ferrite SOFC cathodes with YSZ electrolyte. J Power Sources. 2004;129:188–92. doi:10.1016/j.jpowsour.2003.11.039
Ren Y, Küngas R, Gorte RJ, Deng C. The effect of A-site cation (Ln=La, Pr, Sm) on the crystal structure, conductivity and oxygen reduction properties of Sr-doped ferrite perovskites. Solid State Ionics. 2012;212:47–54. doi:10.1016/j.ssi.2007.12.010
Kharton VV, Kovalevsky AV, Patrakeev MV, Tsipis EV, Viskup AP, Kolotygin VA, Yaremchenko AA, Shaula AL, Kiselev EA, Waerenborgh JC. Oxygen Nonstoichiometry, Mixed Conductivity, and Mӧssbauer Spectra of Ln0.5A0.5FeO3-δ (Ln = La-Sm, A = Sr, Ba): Effects of Cation Size. Chem Mater. 2008;20:6457–67. doi:10.1021/cm801569j
Khvostova LV, Volkova NE, Gavrilova LYa, Cherepanov VA. Crystal structure, oxygen nonstoichiometry and properties of novel Ruddlesden-Popper phase Sm1.8Sr1.2Fe2O7 δ. Mater Lett. 2018;213:158–61. doi:10.1016/j.matlet.2017.11.041
Gavrilova LYa, Aksenova TV, Volkova NE, Podzorova AS, Cherepanov VA. Phase equilibria and crystal structure of the complex oxides in the Ln–Ba–Co–O (Ln=Nd, Sm) systems. J Solid State Chem. 2011;184:2083–7. doi:10.1016/j.jssc.2011.06.006
Khalyavin DD, Sazonov AP, Troyanchuk IO, Szymczak R, Szymczak H. Phase relations in the systems Ln1-xBaxCoO3-δ (0
Maignan A, Martin C, Pelloquin D, Nguyen N, Raveau B. Structural and Magnetic Studies of Ordered Oxygen-Deficient Perovskites LnBaCo2O5+δ, Closely Related to the “112” Structure. J Solid State Chem. 1999;142:247–60. doi:10.1006/jssc.1998.7934
Millange F, Caignaert V, Domengès B, Raveau B. Order-Disorder Phenomena in New LaBaMn2O6-x CMR Perovskites. Crystal and Magnetic Structure. Chem Mater. 1998;10:1974–83. doi:10.1021/cm980130v
Zhou W, Lin CT, Liang WY. Synthesis and Structural Studies of the Perovskite-Related Compound YBaCo2O5+x. Adv Mater. 1993; 5(10)735–8. doi:10.1002/adma.19930051010
Tsvetkov DS, Sereda VV, Zuev AYu. Oxygen nonstoichiometry and defect structure of the double perovskite GdBaCo2O6−δ. Solid State Ionics. 2010;180:1620-5. doi:10.1016/j.ssi.2009.10.014
Anderson PS, Kirk CA, Knudsen J, Reaney IM, West AR. Structural characterization of ReBaCo2O6-δ phases (RE = Pr, Nd, Sm, Eu, Gd, Tb, Dy, Но). Solid State Scien. 2005;7:1149–56. doi:10.1016/j.solidstatesciences.2005.03.004
Seikh MdM, Simon Ch, Caignaert V, Pralong V, Lepetit MB, Boudin S, Raveau B. New Magnetic Transitions in the Ordered Oxygen-Deficient Perovskite LnBaCo2O5.50+δ. Chem Mater. 2008;20:231–8. doi:10.1021/cm7026652
Kim J-H, Kim Y, Connor PA, Irvine J, Bae J, Zhou W. Structural, thermal and electrochemical properties of layered perovskite SmBaCo2O5+d, a potential cathode material for intermediate-temperature solid oxide fuel cells. J Power Sources. 2009;194:704–11. doi:10.1016/j.jpowsour.2009.06.024
Aksenova TV, Gavrilova LYu, Yaremchenko AA, Cherepanov VA, Kharton VV. Oxygen nonstoichiometry, thermal expansion and high-temperature electrical properties of layered NdBaCo2O5+δ and SmBaCo2O5+δ. Mat Res Bull. 2010;45:1288–92. doi:10.1016/j.materresbull.2010.05.004
Sun W, Bi L, Yan L, Peng R, LiuW. Synthesis of SmBaCo2O6−δ powder by the combustion process using Co3O4 as precursor. J Alloys Compd. 2009;481:L40–2. doi:10.1016/j.jallcom.2009.03.150
Zhang K, Ge L, Ran R, Shao Z, Lio S. Synthesis, Characterization and Evaluation of Cation-Ordered LnBaCo2O5+δ as Materials of Oxygen Permeation Membranes and Cathodes of SOFCs. Acta Mater. 2008;56:4876–89. doi:10.1016/j.actamat.2008.06.004
Volkova NE, Gavrilova LYa, Cherepanov VA, Aksenova TV, Kolotygin VA, Kharton VV. Synthesis, crystal structure and properties of SmBaCo2−xFexO5+δ. J Solid State Chem. 2013;204:219–23. doi:10.1016/j.jssc.2013.06.001
Lomakov MV, Istomin SYa, Abakumov AM, Van Tandeloo G, Antipov EV. Synthesis and characterization of oxygen-deficient oxides BaCo1-xYxO3-y, x=0.15, 0.25 and 0.33, with the perovskite structure. Solid State Ionics. 2008;179:1885–9. doi:10.1016/j.ssi.2008.05.004
Siwen L, Yufang R. Studies on the synthetic, structural, electrical, and magnetic properties of the new layered oxides Ln2MCo2O7 (Ln = Sm, Gd; M = Sr, Ba). J Solid State Chem. 1995;114:286–8. doi:10.1006/jssc.1995.1042
Gillie LJ, Hadermann J, Hervieu M, Maignan A, Martin C. Oxygen Vacancy Ordering in the Double-layered Ruddlesden-Popper Cobaltite Sm2BaCo2O7-δ. Chem Mater. 2008;20:6231–7. doi:10.1021/cm8010138
Zhiyu Q, Xianran X, Wenxia Y, Soukun W, Xiaolong C, Jingkui L, Sishen X. Phase relations and compounds in the Sm2O3 – BaO – CuO system at 950°C in air. J Alloys Compd. 1993;202:77–80. doi:10.1016/0925-8388(93)90521-N
Subasri R, Sreedharan OM. Thermodynaqmic stabilities of Ln2BaO4 (Ln = Nd, Sm, Eu or Gd) by CaF2 – based Emf measurements. J Alloys Compd. 1998;274:153–6. doi:10.1016/S0925-8388(98)00548-9
Karen P, Woodward PM, Santhosh PN, Vogt T, Stephens PW, Pagola SJ. Verwey transition under oxygen loading in RBaFe2O5+w (R=Nd and Sm). J Solid State Chem. 2002;167:480–93. doi:10.1006/jssc.2002.9665
Karen P, Woodward PM. Synthesis and structural investigations of the double perovskites REBaFe2O5+w (RE=Nd, Sm). J Mater Chem. 1999;9:789–97. doi:10.1039/A809302D
Moritomo Y, Hanawa M, Ohishi Y, Kato K, Nakamura J, Karppinen M, Yamauchi H. Physical pressure effect on the charge-ordering transition of BaSmFe2O5.0. Phys Rev B. 2003;68:060101(4). doi:10.1103/PhysRevB.68.060101
Elzubair A, El Massalami M, Domingues PH. On the structure and magnetic properties of the series RBa2Fe3O8+x (R = La, Nd, Sm, Gd). Physica B. 1999;271:284–93. doi:10.1016/S0921-4526(99)00205-7
Lindén J, Karen P, Kjekshus A, Miettinen J, Karppinen M. Partial oxygen ordering in cubic perovskite REBa2Fe3O8+w (RE = Gd, Eu, Sm, Nd). J Solid State Chem. 1999;144:398–404. doi:10.1006/jssc.1999.8178
Volkova NE, Lebedev OI, Gavrilova LYa, Turner S, Gauquelin N, Seikh MdM, Caignaert V, Cherepanov VA, Raveau B, van Tendeloo G. Nanoscale Ordering in Oxygen Deficient Quintuple Perovskite Sm2-εBa3+εFe5O15-δ: Implication for Magnetism and Oxygen Stoichiometry. Chem Mater. 2014;26:6303−10. doi:10.1021/cm503276p
Volkova NE, Urusova AS, Gavrilova LYa, Bryuzgina AV, Deryabina KM, Mychinko MYu, Lebedev OI, Raveau B, Cherepanov VA. Special Features of Phase Equilibriums in Ln–Ba–Fe–O Systems. Russian Journal of General Chemistry. 2016;86(8):1800–4. doi:10.1134/S1070363216080041
Itagaki Y, Mori M, Hosoya Y, Aono H, Sadaoka Y. O3 and NO2 sensing properties of
SmFe1-xCoxO3 perovskite oxides. Sens Actuators, B. 2007;122:315–20. doi:10.1016/j.snb.2006.06.001
Zhao M, Peng H, Hu J, Han Z. Effect of Cobalt doping on the microstructure, electrical and ethanol-sensing properties of SmFe1-xCoxO3. Sens Actuators, B. 2008;129:953–7. doi:10.1016/j.snb.2007.10.012
Mori M, Itagaki Y, Sadaoka Y. Effect of VOC on ozone detection using semiconducting sensor with SmFe1-xCoxO3 perovskite-type oxide. Sens Actuators, B. 2012;163:44–50. doi:10.1016/j.snb.2011.12.047
Zhao M, Peng H, Fang S, Hu J. Microstructure, electrical and ethanol-sensing properties of perovskite-type SmFe0.7Co0.3O3. Sens Actuators, B. 2008;130:609–13. doi:10.1016/j.snb.2007.10.017
Zhang R, Hu J, Zhao M, Han Z, Wei J, Wu Z, Qin H, Wang K. Elecctrical and CO-sensing properties of SmFe0.7Co0.3O3 perovskite oxide. Mater Sci Eng, B. 2010;171:139–43. doi:10.1016/j.mseb.2010.03.087
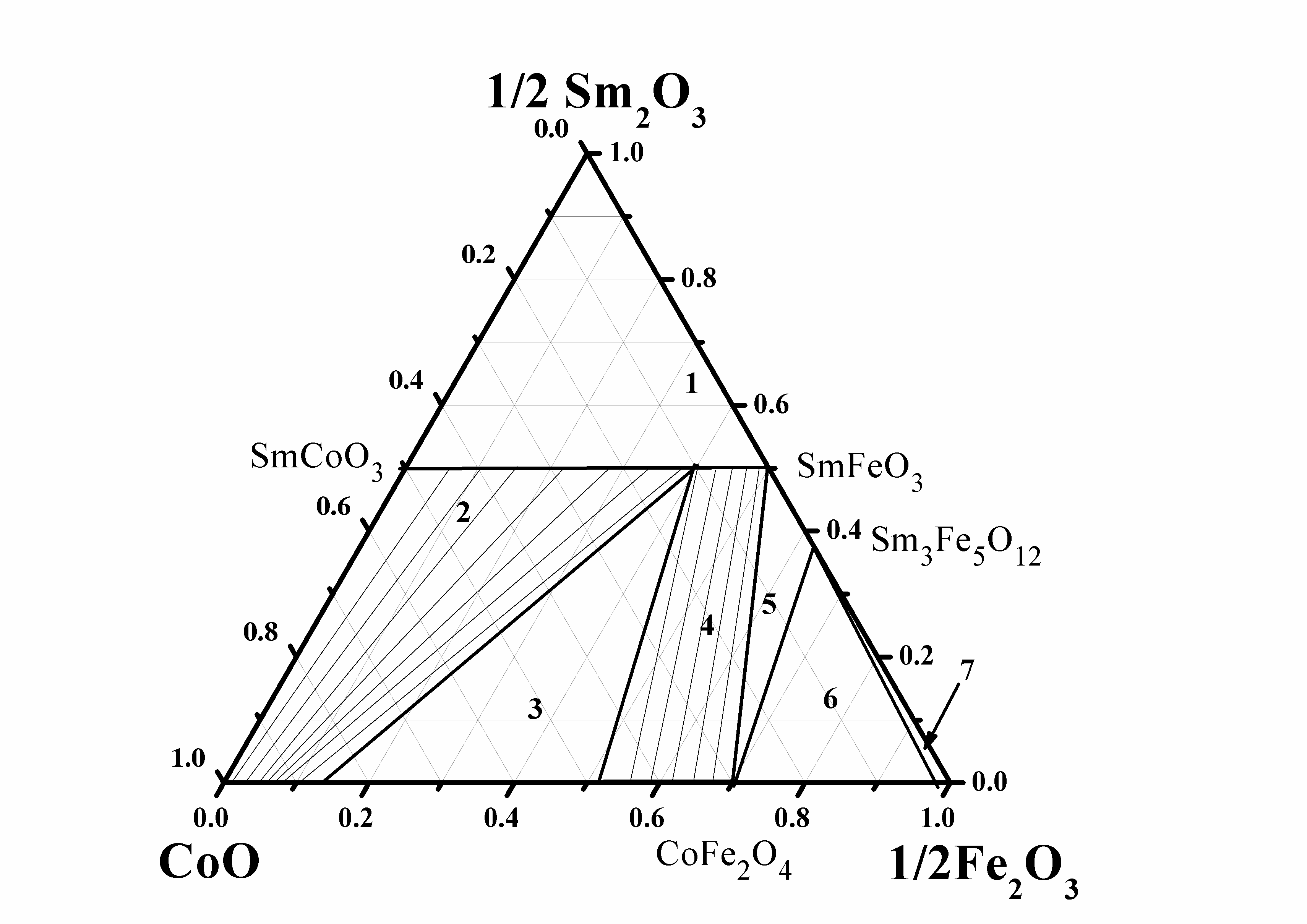
Galayda AP, Volkova NE, Gavrilova LYa, Balymov KG, Cherepanov VA. Phase equilibria, structure and properties of intermediate phases in the Sm2O3 - Fe2O3 - CoO and Sm2O3 - CaO - CoO systems. J Alloys Compd. 2017;718:288–97. doi:10.1016/j.jallcom.2017.05.044
Ben Tahar L, Smiri LS, Artus M, Joudrier A-L, Herbst F, Vaulay MJ, Ammar S, Fiévet F. Characterization and magnetic properties of Sm- and Gd-substituted CoFe2O4 nanoparticles prepared by forced hydrolysis in polyol. Mater Res Bull. 2007;42:1888–96. doi:10.1016/j.materresbull.2006.12.014
Ben Tahar L, Artus M, Ammar S, Smiri LS, Herbst F, Vaulay M-J, Richard V, Grenèche J-M, Villain F, Fiévet F.. Magnetic properties of CoFe1.9RE0.1O4 nanoparticles (RE = La, Ce, Nd, Sm, Eu, Gd, Tb, Ho) prepared in polyol. J Magn Magn Mater. 2008;320:3242 50. doi:10.1016/j.jmmm.2008.06.031
Guo L, Shen X, Song F, Lin L, Zhu Y. Structure and magnetic properties of CoFe2-xSmxO4 (x=0-0.2) nanofibers prepared by sol-gel route. Mater Chem Phys. 2011;129:943–7. doi:10.1016/j.matchemphys.2011.05.023
Ruiz MM, Mietta JL, Antonel PS, Pérez OE, Negri RM, Jorge G. Structural and magnetic properties of Fe2-xCoSmxO4-nanoparticles and Fe2-xCoSmxO4-PDMS magnetoelastomers as a function of Sm content. J Magn Magn Mater. 2013;327:11–9. doi:10.1016/j.jmmm.2012.09.020
Rashad MM, Mohamed RM, El-Shall H. Magnetic properties of nanocrystalline Sm-substituted CoFe2O4 synthesized by citrate precursor method. J Mater Process Technol. 2008;198:139–46. doi:10.1016/j.jmatprotec.2007.07.012
Alifanti M, Bueno G, Parvulescu V, Parvulescu VI, Corberán VC. Oxidation of ethane on high specific surface SmCoO3 and PrCoO3 perovskites. Catal Today. 2009;143:309–14. doi:10.1016/j.cattod.2009.02.026
DOI: https://doi.org/10.15826/chimtech.2018.5.1.04
Copyright (c) 2017 N.E. Volkova, L.V. Khvostova, A.P. Galaida, L.Ya. Gavrilova, V.A. Cherepanov

This work is licensed under a Creative Commons Attribution 4.0 International License.
Chimica Techno Acta, 2014–2025
eISSN 2411-1414
Copyright Notice






