Effect of Alkylation on the Kinetic Stability of Arsenodiester and Organoarsenicals against Hydrolysis: A Theoretical Study
Abstract
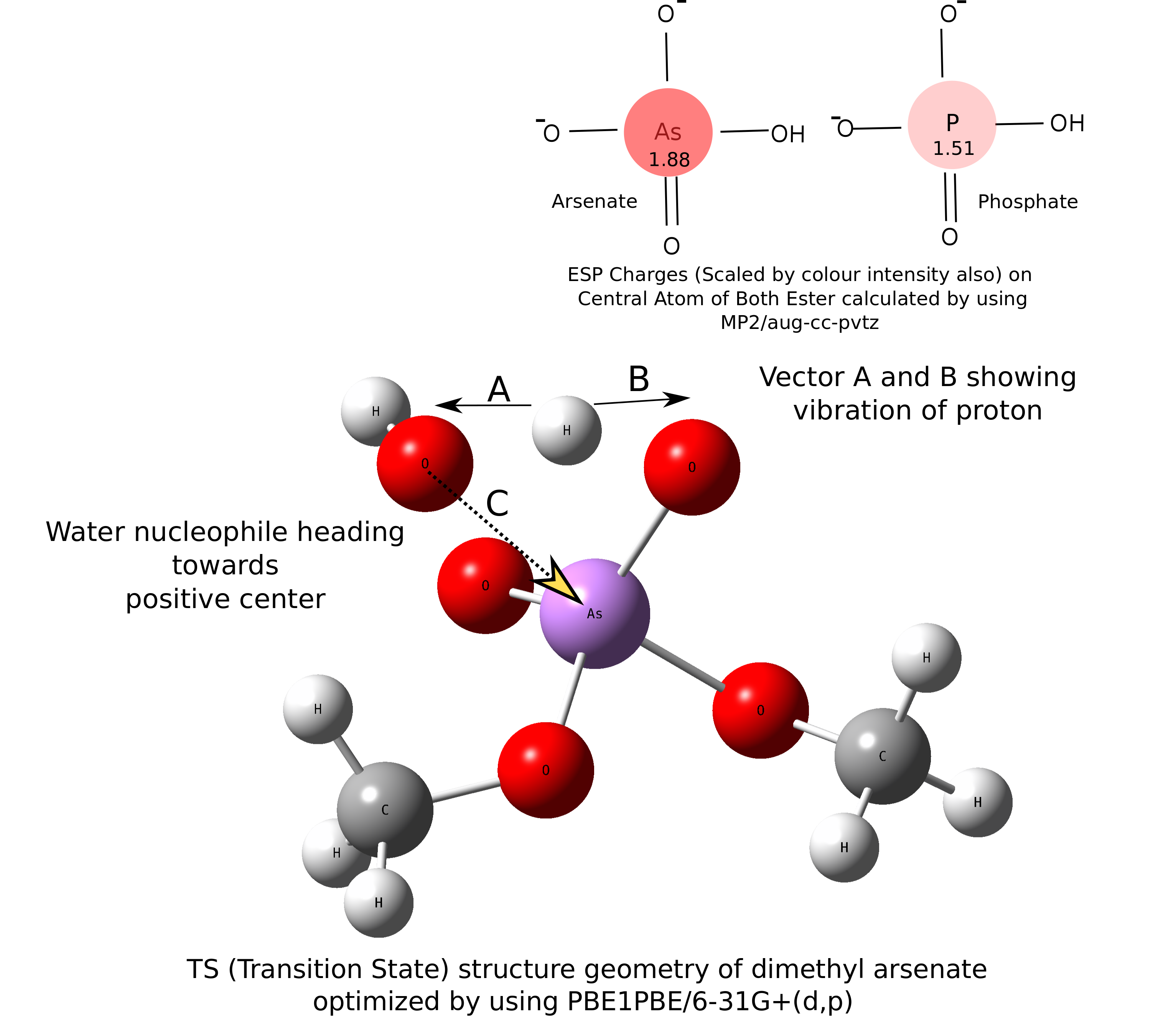
Arsenic diesters have same structural and chemical properties as Pi (phosphate) diester. Beside this structural similarity, arsenate is not considered by cellular processes to replace phosphate. Quantum calculation reveals that this happens due to very high hydrolysis rate of Asi diester (As–O-bond-based compounds) as compared to Pi, but how organoarsenicals (As–C-bond-based compounds) that are produced by alkylation of Asi survive in highly aqueous tissues of marine organisms? We found that this alkylation results in lower hydrolysis rate of Asi diester. Our work concluded that alkylating effort by our body on Asi is to avoid structural ambiguity with phosphate and excrete out arsenic in the form of organoarsenicals from body.
Keywords
Full Text:
PDFReferences
Cullen WR, Reimer KJ. Arsenic speciation in the environment. Chem Rev. 1989;89(4):713-64. doi:10.1021/cr00094a002
Kenney LJ, Kaplan JH. Arsenate substitutes for phosphate in the human red cell sodium pump and anion exchanger. J Biol Chem. 1988;263(17):7954-60.
Westheimer FH. Why nature chose phosphates. Science. 1987;235(4793):1173-8. doi:10.1126/science.2434996
Baer CD, Edwards JO, Rieger PH. Kinetics of the hydrolysis of arsenate (V) triesters. Inorg Chem. 1981;20(3):905-7.
Rehman K, Naranmandura H. Arsenic metabolism and thioarsenicals. Metallomics. 2012;4(9):881-92. doi:10.1039/C2MT00181K
Lagunas R, Pestana D, Diez-Masa JC. Arsenic mononucleotides. Separation by high-performance liquid chromatography and identification with myokinase and adenylate deaminase. Biochemistry. 1984;23(5):955–60. doi:10.1021/bi00300a024
Adams SR, Sparkes MJ, Dixon HBF. The arsonomethyl analogue of adenosine 5′-phosphate. An uncoupler of adenylate kinase. Biochem J. 1984;221(3):829–36. doi:10.1042/bj2210829
Avron M, Jagendorf AT. Evidence concerning the mechanism of adenosine triphosphate formation by spinach chloroplasts. J Biol Chem. 1959;234(4):967–72.
Gresser MJ. ADP-arsenate. Formation by submitochondrial particles under phosphorylating conditions. J Biol Chem. 1981;256(12):5981–3.
Kline PC, Schramm VL. Purine nucleoside phosphorylase. Catalytic mechanism and transition-state analysis of the arsenolysis reaction. Biochemistry. 1993;32(48):13212–9. doi:10.1021/bi00211a033
Mládek A, Šponer J, Sumpter BG, Fuentes-Cabrera M, Šponer JE. Theoretical modeling on the kinetics of the arsenate-ester hydrolysis: implications to the stability of As-DNA. Phys Chem Chem Phys. 2011;13(23):10869-71. doi:10.1039/C1CP20423H
Schroeder GK, Lad C, Wyman P, Williams NH, Wolfenden R. The time required for water attack at the phosphorus atom of simple phosphodiesters and of DNA. Proc Natl Acad Sci USA. 2006;103(11):4052-5. doi:10.1073/pnas.0510879103
Ribeiro AJ, Ramos MJ, Fernandes PA. Benchmarking of DFT functionals for the hydrolysis of phosphodiester bonds. J Chem Theory Comput. 2010;6(8):2281-92. doi:10.1021/ct900649e
Miertuš S, Scrocco E, Tomasi J. Electrostatic Interaction of a Solute with a Continuum. A Direct Utilization of ab initio Molecular Potentials for the Prevision of Solvent Effects. Chem Phys. 1981;55:117-29. doi:10.1016/0301-0104(81)85090-2
Fukui K. The path of chemical reactions-the IRC approach. Acc Chem Res. 1981;14(12):363-8. doi:10.1021/ar00072a001
Ochterski JW. Thermochemistry in Gaussian [Internet]. c2000 [cited 2018 June 14]. Available from: http://gaussian.com/thermo/.
Martin F, Zipse H. Charge distribution in the water molecule—a comparison of methods. J Comput Chem. 2005;26(1):97-105. doi:10.1002/jcc.20157
DOI: https://doi.org/10.15826/chimtech.2018.5.2.01
Copyright (c) 2018 Boota Singh, Rohan Ranjan Waliya, Sougata Santra, Kousik Giri, Grigory V. Zyryanov

This work is licensed under a Creative Commons Attribution 4.0 International License.
Chimica Techno Acta, 2014–2025
eISSN 2411-1414
Copyright Notice






