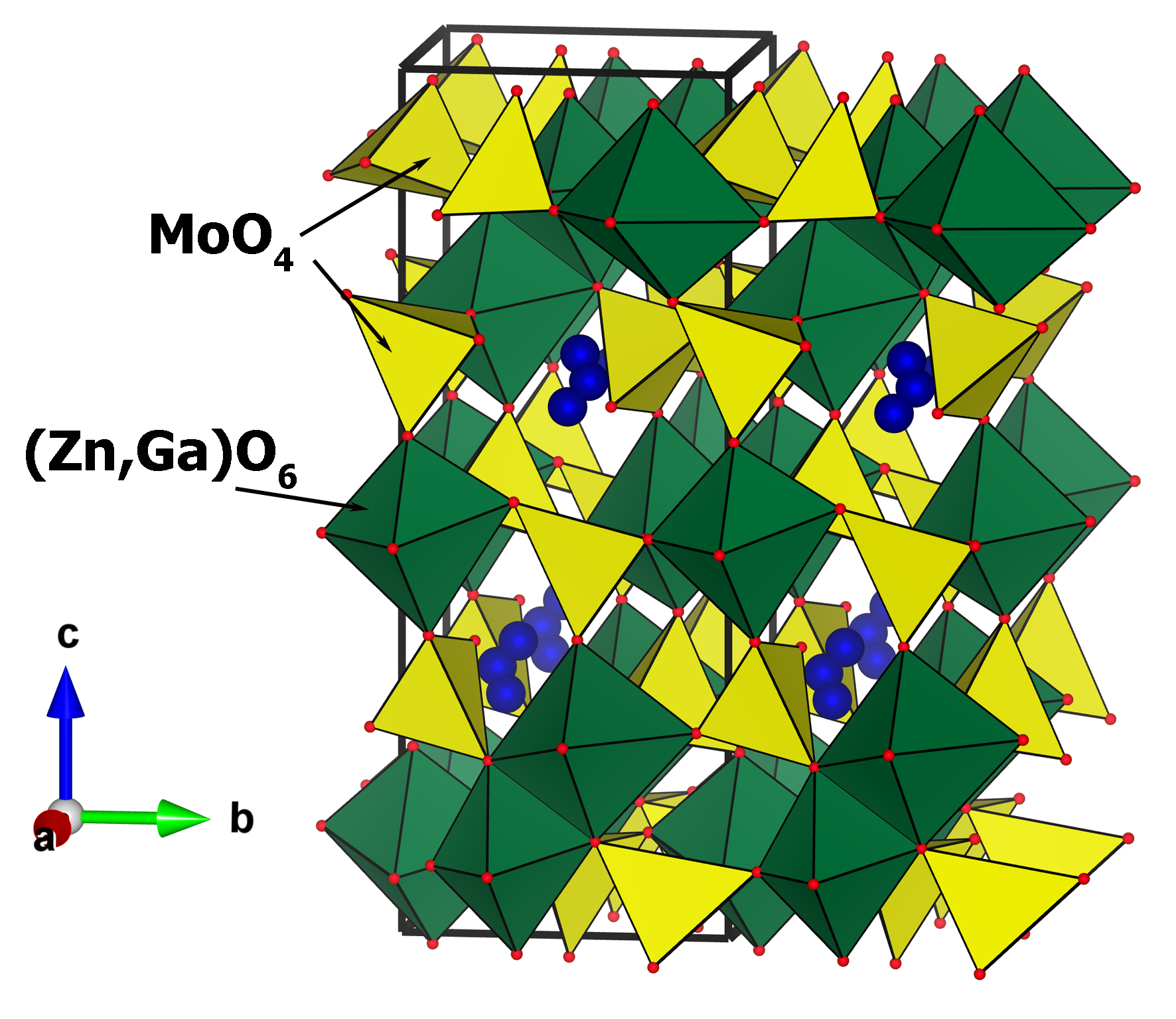
Synthesis, crystal structure and electrophysical properties of triple molybdates containing silver, gallium and divalent metals
Abstract
Keywords
Full Text:
PDFReferences
Kotova IYu. Phase formation in the Ag2MoO4–CoMoO4–Al2(MoO4)3 system. Russ J Inorg Chem. 2014;59:844–8. doi:10.7868/S0044457X14080133
Kotova IYu, Korsun VP. Phase in the Ag2MoO4–MgMoO4–Al2(MoO4)3. Russ J Inorg Chem. 2010;55:955–8. doi: 10.1134/S0036023610060203
Kotova IYu, Korsun VP. Phase formation in the system involving silver, magnesium, and indium molybdates, Russ J Inorg Chem 2010;55:1965–9. doi: 10.1134/S0036023610120247
Kotova IYu, Belov DA, Stefanovich SYu. Ag1–xMg1–xR1+x(MoO4)3 Ag+-conducting NASICON-like phases, where R = Al or Sc and 0 ≤ x ≤ 0.5. Russ J Inorg Chem 2011;56:1189−93. doi: 10.1134/S0036023611080122
Bouzidi C, Frigui W, Zid MF. Synthèse et structure crystalline d'un matériau noir AgMnII3(MnIII0.26Al0.74)(MoO4)5. Acta Cryst. 2015;E71:299–304. doi: 10.1107/S2056989015003345
Nasri R, Chérif SF, Zid MF. Structure cristalline de la triple molybdate Ag0.90Al1.06Co2.94(MoO4)5. Acta Cryst. 2015; E71:388−91. doi: 10.1107/s2056989015005290
Kotova IYu, Solodovnikov SF, Solodovnikova ZA, Belov DA, Stefanovich SYu, Savina AA, Khaikina EG. New series of triple molybdates AgA3R(MoO4)5 (A = Mg, R = Cr, Fe; A = Mn, R = Al, Cr, Fe, Sc, In) with framework structures and mobile silver ion sublattices. J Solid State Chem. 2016;238:121–8. doi: 10.1016/j.jssc.2016.03.003
Balsanova LV. Synthesis of crystals of silver containing oxide phases based on molybdenum, study of their structure and properties. Vestnik VSGUTU. 2015;5:63−9.
Kotova IYu, Savina AA, Khaikina EG. Crystal structure of new triple molybdate AgMg3Ga(MoO4)5 from Rietveld refinement. Powder Diffraction. 2017;32(4):255–60. doi: 10.1017/S0885715617000811
Yanushkevich TM, Zhukovsky VM, Ustyantsev VM. Fazovaya diagramma sistemy NiO‒МоОз. [Phase diagram of the system NiO‒МоОз]. Russ J Inorg Chem. 1974;19(7):1932–6.
Zhukovsky VM, Tkachenko EV. Fazovye ravnovesiya v molibdatnykh sistemakh. [Phase equilibria in molybdate systems]. Zbornik nauchnykh trudov. “Fizicheskaya khimiya okislov” Moscow: Nauka; 1981.106–15. Russian
Reichelt W. Weber T, Soehnel T. at al. Electronic structure and luminescence mechanisms in ZnMoO4 crystals. J Phys: Condes Mater. 2011;23:244‒59.
Tsyrenova GD, Bazarova JG, Mokhosoev MV. Phase equilibria in systems МеО‒МоО3 (Me ‒ Mg, Мп, Zn). Russ J Inorg Chem. 1986;31(12):3120–3.
Rajaram P, Viswanathan B, Aravamudan G. Studies on the formation of manganese molybdates. Thermochim acta. 1973;7(2):123–9.
Kohlmuller R, Faurie J.-P. Etude des systemes MoO3–Ag2MoO4 et MoO3–MO (M – Cu, Zn, Cd). Bull Soc chim. France. 1968;11:4379–82.
Larson AC, Von Dreele RB. General Structure Analysis System (GSAS) (Report LAUR 86-748). 2004. Los Alamos, New Mexico: Los Alamos National Laboratory.
Shannon RD. Revised effective ionic radii and systematic studies of interatomic distances in dalides and chalcogenides. Acta Cryst. 1976;A32:751–67. doi:10.1107/S0567739476001551
Gates Stacy D, Julie A, Lind C. Gates Non-hydrolytic sol-gel synthesis, properties, and high-pressure behavior of gallium molybdate. J Mater Chem. 2006;16:4214–9. doi:10.1039/B608864C
Perepelitsa AP, Golub AM, Badayev YuB, Shapoval VN. Double molybdates of aluminum, gallium, indium, chromium, iron and bismuth with monovalent silver and thallium. Russ J Inorg Chem. 1977;22(4):994–7.
Klevtsova RF, Vasiliev AD, Kozhevnikova NM, Glinskaya LA, Kruglik AI, Kotova IYu. Synthesis and crystal structural study of ternary molybdate NaMg3In(MoO4)5. J Struct Chem. 1994;34:784−8.
Rietveld HM. A profile refinement method for nuclear and magnetic structures. J Appl Cryst. 1969;2:65–71.
DOI: https://doi.org/10.15826/chimtech.2018.5.3.02
Copyright (c) 2018 Irina Kotova, Aleksandra Savina, Alena Vandysheva, Dmitry Belov, Sergey Stefanovich

This work is licensed under a Creative Commons Attribution 4.0 International License.
Chimica Techno Acta, 2014–2025
eISSN 2411-1414
Copyright Notice







