Arsenate and Arsenite Reaction Kinetics with Ferric Hydroxides Using Quantum Chemical Calculations
Abstract
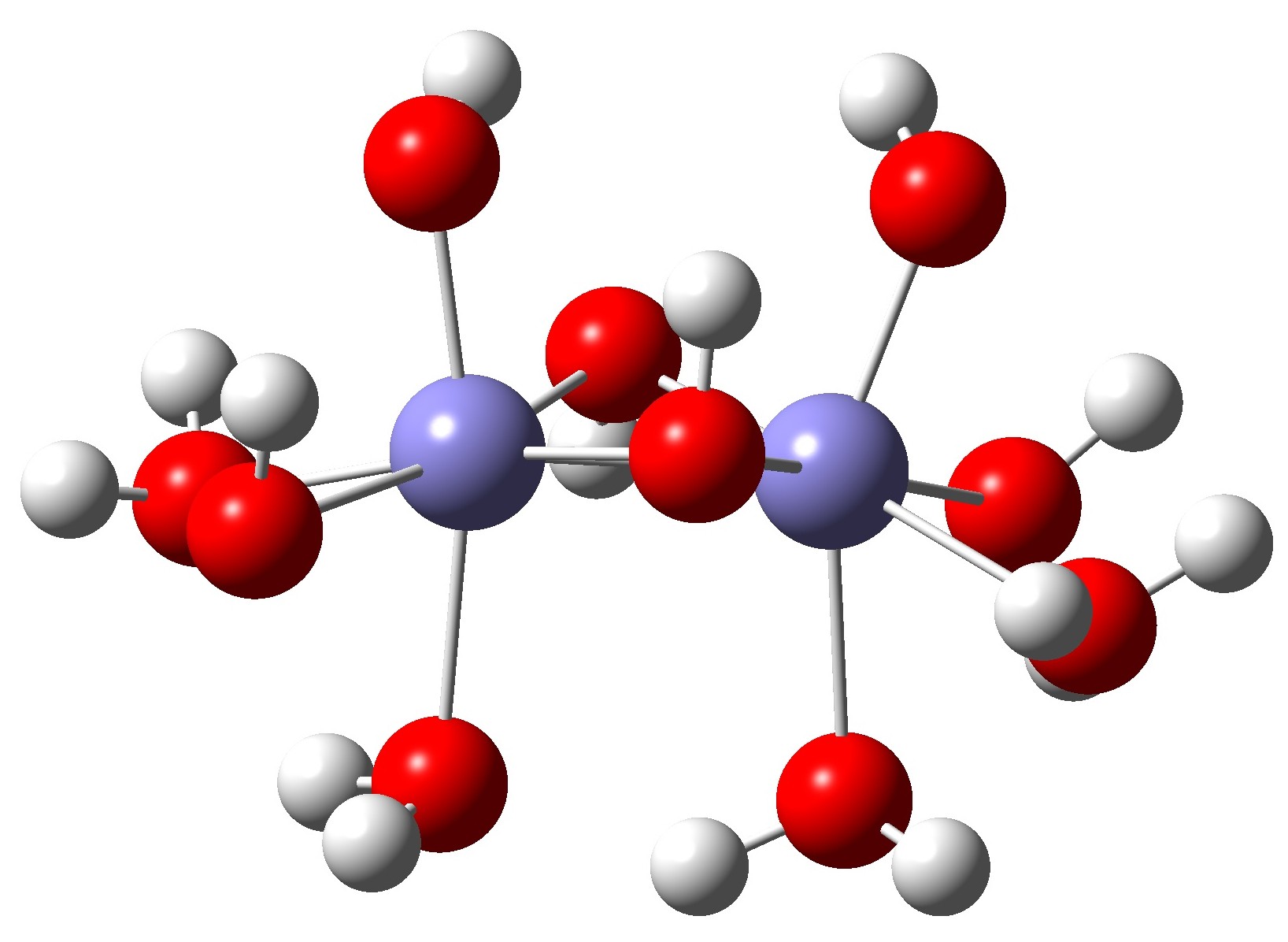
The knowledge of the mechanism involved in the process of adsorption and desorption of arsenate and arsenite with ferric hydroxides is important to address the water toxicity problems and to tackle the adverse effect of these substances in nature. An essential outcome of previous studies on the kinetics of the arsenate adsorption on aluminum and iron oxide was that the adsorption is a two-phase (bi-phase) process. Quantum mechanical calculations using density functional theory were used to determine the thermodynamic variables governing the adsorption process to get an insight into the stability of the complexes formed. The previous investigation showed that the positively charged ferric hydroxide cluster had better stability at neutral pH. The chemisorbed charged monodentate complexes had Gibbs free energy of reaction -55.97 kcal/mol where the bidentate complex formation had Gibbs free energy of reaction -62.55 kcal/mol. The bidentate complex having a negative charge had more Gibbs free energy of reaction compared to uncharged one. The results of the study indicate that Gibbs free energy for the reaction has a significant role in controlling the kinetics of the adsorption and sorption process of arsenate on ferric hydroxide clusters.
Keywords
Full Text:
PDFReferences
Bhumbla DK, Keefer RF. Arsenic mobilization and bioavailability in soils. In: Arsenic in the Environment; Part I: Cycling and Characterization; Nriagu JO, Editor. John Wiley & Sons: New York, 1994. pp 51−82.
Manning BA, Fendorf SE, Goldberg S. Surface Structures and Stability of Arsenic(III) on Goethite: Spectroscopic Evidence for Inner-Sphere Complexes. Environmental Science & Technology. 1998;32(16):2383-8. doi:10.1021/es9802201
Waychunas GA, Davis JA, Fuller CC. Geometry of sorbed arsenate on ferrihydrite and crystalline FeOOH: Re-evaluation of EXAFS results and topological factors in predicting sorbate geometry, and evidence for monodentate complexes. Geochimica et Cosmochimica Acta. 1995;59(17):3655-61. doi:10.1016/0016-7037(95)00276-6
Waychunas GA, Rea BA, Fuller CC, Davis JA. Surface chemistry of ferrihydrite: Part 1. EXAFS studies of the geometry of coprecipitated and adsorbed arsenate. Geochimica et Cosmochimica Acta. 1993;57(10):2251-69. doi:10.1016/0016-7037(93)90567-G
Grossl PR, Eick M, Sparks DL, Goldberg S, Ainsworth CC. Arsenate and Chromate Retention Mechanisms on Goethite. 2. Kinetic Evaluation Using a Pressure-Jump Relaxation Technique. Environmental Science & Technology. 1997;31(2):321-6. doi:10.1021/es950654l
Kubicki JD. Comparison of As(III) and As(V) Complexation onto Al- and Fe-Hydroxides. Advances in Arsenic Research. ACS Symposium Series. 915: American Chemical Society; 2005. p. 104-17.
Sherman DM, Randall SR. Surface complexation of arsenic(V) to iron(III) (hydr)oxides: structural mechanism from ab initio molecular geometries and EXAFS spectroscopy. Geochimica et Cosmochimica Acta. 2003;67(22):4223-30. doi:10.1016/S0016-7037(03)00237-0
Zhang H, Selim HM. Kinetics of Arsenate Adsorption−Desorption in Soils. Environmental Science & Technology. 2005;39(16):6101-8. doi:10.1021/es050334u
Fuller CC, Davis JA, Waychunas GA. Surface chemistry of ferrihydrite: Part 2. Kinetics of arsenate adsorption and coprecipitation. Geochimica et Cosmochimica Acta. 1993;57(10):2271-82. doi:10.1016/0016-7037(93)90568-H
Yang W, Zhao N, Zhang N, Chen W, Kan AT, Tomson MB. Time-dependent adsorption and resistant desorption of arsenic on magnetite nanoparticles: kinetics and modeling. Desalination and Water Treatment. 2012;44(1-3):100-9. doi:10.1080/19443994.2012.691808
Raven KP, Jain A, Loeppert RH. Arsenite and Arsenate Adsorption on Ferrihydrite: Kinetics, Equilibrium, and Adsorption Envelopes. Environmental Science & Technology. 1998;32(3):344-9. doi:10.1021/es970421p
Luengo C, Brigante M, Avena M. Adsorption kinetics of phosphate and arsenate on goethite. A comparative study. Journal of Colloid and Interface Science. 2007;311(2):354-60. doi:https://doi.org/10.1016/j.jcis.2007.03.027
Austin A, Petersson GA, Frisch MJ, Dobek FJ, Scalmani G, Throssell K. A Density Functional with Spherical Atom Dispersion Terms. Journal of Chemical Theory and Computation. 2012;8(12):4989-5007. doi:10.1021/ct300778e
Gaussian09, Revision D.01, Gaussian, Inc., Wallingford CT, 2013
Zhao Y, Truhlar DG. The M06 suite of density functionals for main group thermochemistry, thermochemical kinetics, noncovalent interactions, excited states, and transition elements: two new functionals and systematic testing of four M06-class functionals and 12 other functionals. Theoretical Chemistry Accounts. 2008;120(1):215-41. doi:10.1007/s00214-007-0310-x
Weigend F, Ahlrichs R. Balanced basis sets of split valence, triple zeta valence and quadruple zeta valence quality for H to Rn: Design and assessment of accuracy. Phys Chem Chem Phys. 2005;7(18):3297-305. doi:10.1039/B508541A
Farrell J, Chaudhary BK. Understanding Arsenate Reaction Kinetics with Ferric Hydroxides. Environmental Science & Technology. 2013;47(15):8342-7. doi:10.1021/es4013382
DOI: https://doi.org/10.15826/chimtech.2018.5.03.03
Copyright (c) 2018 Cijin J. George, Sougata Santra, G. V. Zyryanov, Kousik Giri

This work is licensed under a Creative Commons Attribution 4.0 International License.
© Website Chimica Techno Acta, 2014–2024
ISSN 2411-1414 (Online)
This journal is licensed under a Creative Commons Attribution 4.0 International






