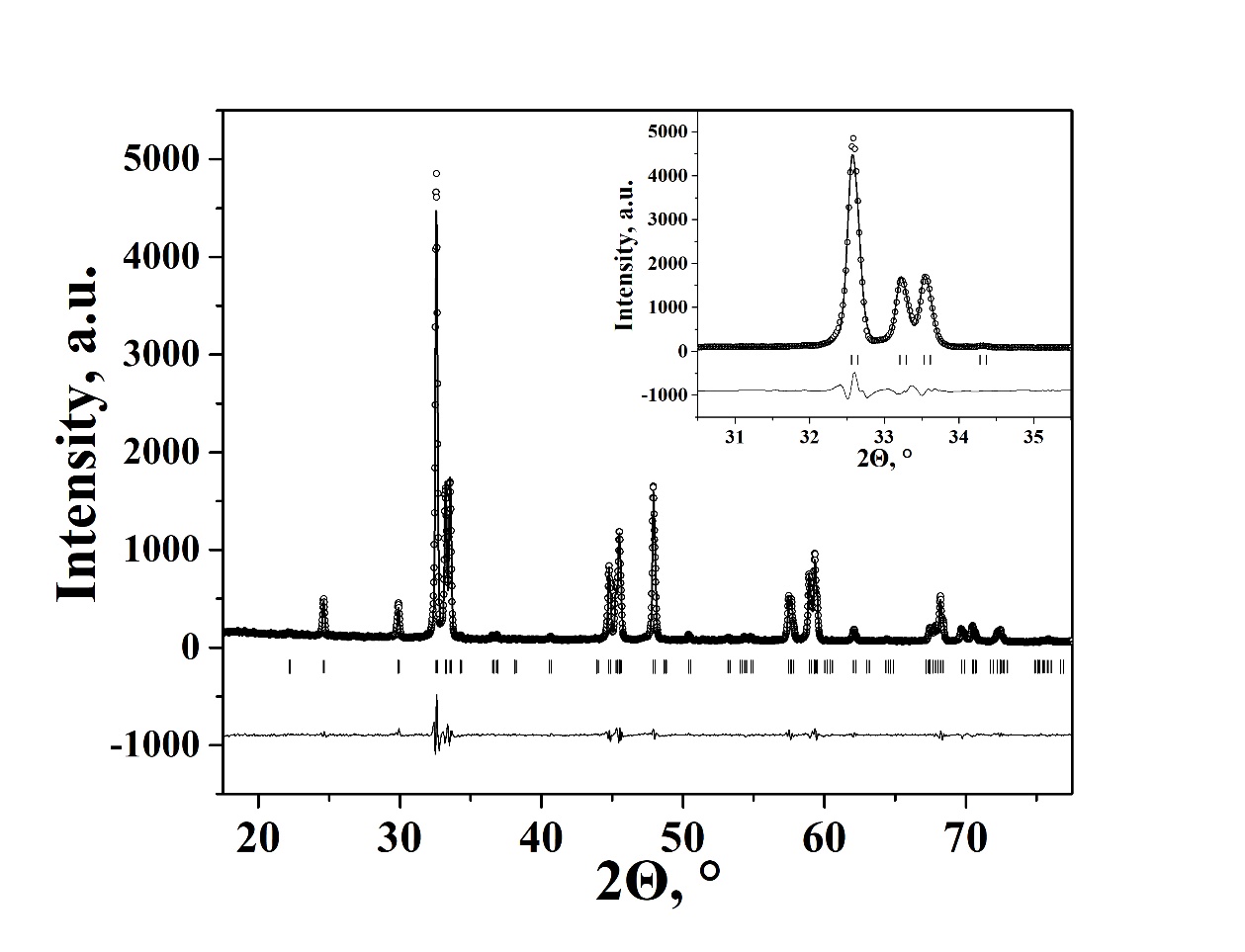
Crystal structure and properties of novel oxide Sm0.9Ca1.1Fe0.7Co0.3O4-d
Abstract
Keywords
Full Text:
PDFReferences
Istomin SYa, Antipov EV. Cathode materials based on perovskite-like transition metal oxides for intermediate temperature solid oxide fuel cells. Russ Chem Rev. 2013;82(7):686–700. doi:10.1070/RC2013v082n07ABEH004390
Dailly J, Fourcade S, Largeteau A, Mauvy F, Grenier JC, Marrony M. Perovskite and A2MO4-type oxides as new cathode materials for protonic solid oxide fuel cells. Electrochim Acta. 2010;55(20):5847–53. doi:10.1016/j.electacta.2010.05.034
Zhao F, Wang X, Wang Z, Peng R, Xia C. K2NiF4 type La2−xSrxCo0.8Ni0.2O4+δ as the cathodes for solid oxide fuel cells. Solid State Ionics. 2008;179:1450–3. doi:10.1016/j.ssi.2008.06.019
Daroukh MAl, Vashook VV, Ullmann H, Tietz F, Arual Raj I. Oxides of the AMO3 and A2MO4-type: structural stability, electrical conductivity and thermal expansion. Solid State Ionics. 2003;158:141–50. doi:10.1016/S0167-2738(02)00773-7
Vashook VV, Yushkevich II, Kokhanovsky LV, Makhnach LV, Tolochko SP, Kononyuk IF, Ullmann H, Altenburg H. Composition and conductivity of some nickelates. Solid State Ionics. 1999;119:23–30. doi:10.1016/S0167-2738(98)00478-0
Ling Z, Xuezhong W, Cunzhen L. Catalytic combustion of diesel soot over K2NiF4-type oxides La2-xKxCuO4. J Rare Earths. 2008.;26(2):254–7. doi:10.1016/S1002-0721(08)60076-9
Skinner SJ. Characterisation of La2NiO4+δ using in-situ high temperature neutron powder diffraction. Solid State Sci. 2003;5:419–26. doi:10.1016/S1293-2558(03)00050-5
Taguchi H, Nakade K, Hirota K. Synthesis and characterization of K2NiF4-type CaLnCoO4 (Ln = Sm and Gd). Mater Res Bull. 2007;42:649–56. doi:10.1016/j.materresbull.2006.08.004
Taguchi H, Kido H, Tabata T. Relationship between crystal structure and electrical property of K2NiF4-type (Ca1 xNd1+x)CoO4-. Physica B. 2004;344:271–7. doi:10.1016/j.physb.2003.09.270
Galayda AP, Volkova NE, Gavrilova LYa, Balymov KG, Cherepanov VA. Phase equilibria, structure and properties of intermediate phases in the Sm2O3-Fe2O3-CoO and Sm2O3-CaO-CoO systems. J Alloys Compd. 2017;718:288–97. doi:10.1016/j.jallcom.2017.05.044
Romero de Paz J, Fernández-Dı́az MT, Hernández Velasco J, Sáez Puche R, Martinez JL. Crystal and Magnetic Structure of PrCaCrO4. J Solid State Chem. 1999;142(1):29–32. doi:10.1006/jssc.1998.7973
Thorogood GJ, Orain P-Y, Ouvry M, Piriou B, Tedesco T, Wallwork KS, Herrmann J, James M. Structure, crystal chemistry and magnetism of rare earth calcium-doped cobaltates: Ln2 xCaxCoO4+δ (Ln=Pr, Nd, Sm, Eu, Gd). Solid State Sci. 2011;13:2113-23. doi:10.1016/j.solidstatesciences.2011.08.008
Volkova NE, Maklakova AV, Gavrilova LYa, Cherepanov VA. Phase equilibria, crystal structure, and properties of intermediate oxides in the Sm2O3–SrO–CoO system. Eur J Inorg Chem. 2017:3285–92. doi:10.1002/ejic.201700321
Nguyen-Trut-Dinh MM, Vlasse M, Perrin M, Le Flem G. Un oxide magnetique bidimensionnel: CaLaFeO4. J Solid State Chem. 1980;32:1–8. doi:10.1016/0022-4596(80)90262-5
Volkova NE, Gavrilova LYa, Cherepanov VA, Aksenova TV, Kolotygin VA, Kharton VV. Synthesis, crystal structure and properties of SmBaCo2-xFexO5+δ. J Solid State Chem. 2013:204;219–23. doi:10.1016/j.jssc.2013.06.001
Jennings AJ, Skinner SJ, Helgason Ö. Structural properties of LaxSr2-xFeO4± at high temperature and under reducing conditions. J Solid State Chem. 2003;175:207–17. doi:10.1016/S0022-4596(03)00248-2
Ki-Woog S, Ki-Tae L. Characterization of NdSrCo1-xFexO4+d (0≤x≤1.0) intergrowth oxide cathode materials for intermediate temperature solid oxide fuel cells. Ceram Int. 2011;37:573–7. doi:10.1016/j.ceramint.2010.10.004
Aksenova TV, Vakhromeeva AE, Elkalashy ShI, Urusova AS, Cherepanov VA. Phase equilibria, crystal structure, oxygen nonstoichiometry and thermal expansion of complex oxides in the Nd2O3 – SrO – Fe2O3 system. J Solid State Chem. 2017;251:70–8. doi:10.1016/j.jssc.2017.04.015
Singh S, Singh D. Effect of increasing Sr content on structural and physical properties of K2NiF4-type phase GdSrFeO4. Ceram Int. 2017;43:3369–76. doi:10.1016/j.ceramint.2016.11.182
Cottrell TL. The Strengths of Chemical Bonds. 2nd ed. London: Butterworth; 1958. 317 p.
Tai L-W, Nasrallah MM, Anderson HU, Sparlin DM, Sehlin SR. Structure and electrical properties of La1-xSrxCo1-yFeyO3. Part 1. The system La0.8Sr0.2Co1-yFeyO3. Solid State Ionics. 1995;76:259–71. doi:10.1016/0167-2738(94)00244-m
Tsipis EV, Kharton VV. Electrode materials and mechanisms in solid oxide fuel cells: brief review. J Solid State Electrochem. 2008;12(11):1367–91. doi:10.1007/s10008-008-0611-6
Pikalova EYu, Murashkina AA, Maragou VI, Demin AK, Strekalovsky VN, Tsiakaras PE. CeO2 based materials doped with lanthanides for applications in intermediate temperature electrochemical devices. Int J Hydrogen Energy. 2011;36:6175–83. doi:10.1016/j.ijhydene.2011.01.132
DOI: https://doi.org/10.15826/chimtech.2018.5.4.01
Copyright (c) 2018 Anastasia P. Galayda, Nadezhda E. Volkova, Anastasia I. Dyagileva, Ludmila Ya. Gavrilova, Vladimir A. Cherepanov, Peter D. Battle

This work is licensed under a Creative Commons Attribution 4.0 International License.
Chimica Techno Acta, 2014–2025
eISSN 2411-1414
Copyright Notice







