Manipulating the grain boundary properties of BaCeO3-based ceramic materials through sintering additives introduction
Abstract
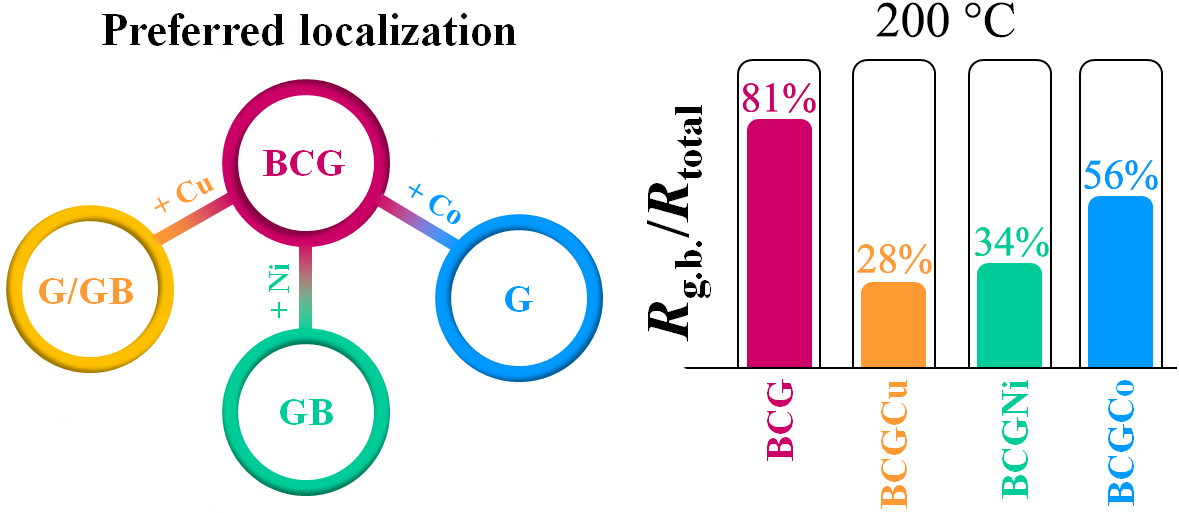
BaCeO3-based materials represent a well-known family of proton-conducting electrolytes, which can be used in different solid oxide electrochemical devices. An effective operation of the latter across an intermediate-temperature range requires improved transport of PCEs, including their grain (G) and grain boundary (GB) components. In the present work, some 3d-elements in a small amount were used as sintering additives to verify the possibility of improving the GB conductivity of BaCe0.9Gd0.1O3–δ. It is shown that copper oxide (CuO) can be considered as one of the most effective sintering agents, since its use enables decreasing the GB density of the BCG ceramic material at the reduced sintering temperatures. The obtained results form a new tactic for designing new protonic electrolytes, whose conductivity might be prevail over ones containing Ni-based modifiers.
Keywords
Full Text:
PDFReferences
Meng Y, Gao J, Zhao Z, Amoroso J, Tong J, Brinkman KS. Review: recent progress in low-temperature proton-conducting ceramics. J Mater Sci. 2019;54(13):9291–312. doi:10.1007/s10853-019-03559-9
Kim J, Sengodan S, Kim S, Kwon O, Bu Y, Kim G. Proton conducting oxides: A review of materials and applications for renewable energy conversion and storage. Renew Sustain Energy Rev. 2019;109:606–18 doi:10.1016/j.rser.2019.04.042
Mohd Rashid NLR, Samat AA, Jais AA, Somalu MR, Muchtar A, Baharuddin NA, Wan Isahak WNR. Review on zirconate-cerate-based electrolytes for proton-conducting solid oxide fuel cell. Cerm Int. 2019;45(6)6605–15. doi:10.1016/j.ceramint.2019.01.045
Putilov LP, Tsidilkovski VI. Impact of bound ionic defects on the hydration of acceptor-doped proton-conducting perovskites. Phys Chem Chem Phys. 2019;21(12):6391–406. doi:10.1039/C8CP07745B
Wang W, Medvedev D, Shao Z. Gas humidification impact on the properties and performance of perovskite‐type functional materials in proton‐conducting solid oxide cell. Adv Funct Mater. 2018;28(48):1802592. doi:10.1002/adfm.201802592
Putilov LP, Demin AK, Tsidilkovski VI, Tsiakaras P. Theoretical modeling of the gas humidification effect on the characteristics of proton ceramic fuel cells. Appl Energy. 2019;242:1448–59. doi:10.1016/j.apenergy.2019.03.096
Tarutin A, Lyagaeva J, Farlenkov A, Plaksin S, Vdovin G, Demin A, Medvedev D. A reversible protonic ceramic cell with symmetrically designed Pr2NiO4+δ-based electrodes: fabrication and electrochemical features. Materials. 2019;12(1):118. doi:10.3390/ma12010118
Danilov N, Lyagaeva J, Vdovin G, Medvedev D. Multifactor performance analysis of reversible solid oxide cells based on proton-conducting electrolytes. Appl Energy. 2019;237:924–34. doi:10.1016/j.apenergy.2019.01.054
Volkov A, Gorbova E, Vylkov A, Medvedev D, Demin A, Tsiakaras P. Design and applications of potentiometric sensors based on proton-conducting ceramic materials. A brief review. Sens Actuators B. 2017;244:1004–15. doi:10.1016/j.snb.2017.01.097
Dai H, Kou H, Wang H, Bi L. Electrochemical performance of protonic ceramic fuel cells with stable BaZrO3-based electrolyte: A mini-review. Electrochem Commun. 2018;96:11–5. doi:10.1016/j.elecom.2018.09.00
Lei Yang, Shizhong Wang, Kevin Blinn, Mingfei Liu, Ze Liu, Zhe Cheng, Meilin Liu. Enhanced sulfur and coking tolerance of a mixed ion conductor for SOFCs: BaZr0.1Ce0.7Y0.2–xYbxO3–δ. Science. 2009;326(5949):126–9. doi:10.1126/science.1174811
Lee YH, Chang I, Cho GY, Park J, Yu W, Tanveer WH, Cha SW. Thin film solid oxide fuel cells operating below 600°C: a review. Int J Precis Eng Manuf. 2018;5(3):441–53. doi:10.1007/s40684-018-0047-0
Cho GY, Lee YH, Cha SW. Thin film process for thin film solid oxide fuel cells - a review. J Korean Soc Precis Eng. 2018;35(12):1119–29. doi:10.7736/KSPE.2018.35.12.1119
Kjølseth C, Fjeld H, Prytz Ø, Dahl PI, Estournès C, Haugsrud R, Norby T. Space-charge theory applied to the grain boundary impedance of proton conducting BaZr0.9Y0.1O3−δ. Solid State Ionics. 2010;181(5–7):268–75. doi:10.1016/j.ssi.2010.01.014
German RM, Suri P, Park SJ. Review: liquid phase sintering. J Mater Sci. 2009;44(1):1–39. doi:10.1007/s10853-008-3008-0
Tong J, Clark D, Bernau L, Subramaniyan A, O'Hayre R. Proton-conducting yttrium-doped barium cerate ceramics synthesized by a cost-effective solid-state reactive sintering method. Solid State Ionics. 2010;181(33–34):1486–98. doi:10.1016/j.ssi.2010.08.022
Yun DS, Kim J, Kim S-J, Lee J-H, Kim J-N, Yoon HC, Yu JH, Kwak M, Yoon H, Cho Y, Yoo C-Y. Structural and electrochemical properties of dense yttria-doped barium zirconate prepared by solid-state reactive sintering. Energies. 2018;11(11):3083. doi:10.3390/en11113083
Fang S, Wang S, Brinkman KS, Su Q, Wang H, Chen F. Relationship between fabrication method and chemical stability of Ni–BaZr0.8Y0.2O3−δ membrane. J Power Sources. 2015;278:614–22. doi:10.1016/j.jpowsour.2014.12.108
Narendar N, Mather GC, Diasa PAN, Fagg DP. The importance of phase purity in Ni–BaZr0.85Y0.15O3−δ cermet anodes – novel nitrate-free combustion route and electrochemical study. RSC Adv. 2013;3(3):859–69. doi:10.1039/C2RA22301E
Han D, Otani Y, Noda Y, Onishi T, Majima M, Uda T. Strategy to improve phase compatibility between proton conductive BaZr0.8Y0.2O3−δ and nickel oxide. RSC Adv. 2016;6(23):19288–97. doi:10.1039/C5RA26947D
Ananyev M, Medvedev D, Gavrilyuk A, Mitri S, Demin A, Malkov V, Tsiakaras P. Cu and Gd co-doped BaCeO3 proton conductors: experimental vs SEM image algorithmic-segmentation results. Electrochim Acta. 2014;125:371–9. doi:10.1016/j.electacta.2013.12.161
Kreuer KD. Proton-conducting oxides. Annu Rev Mater Res. 2003;33:333–59. doi:10.1146/annurev.matsci.33.022802.091825
Malavasi L, Fisher CAJ, Islam MS. Oxide-ion and proton conducting electrolyte materials for clean energy applications: structural and mechanistic features. Chem Soc Rev. 2010;39(11):4370–87. doi:10.1039/B915141A
Kochetova N, Animitsa I, Medvedev D, Demin A, Tsiakaras P. Recent activity in the development of proton-conducting oxides for high-temperature applications. RSC Adv. 2016;6(77):73222–68. doi:10.1039/C6RA13347A
Lander JJ. The phase system BaO–NiO. J Am Chem Soc. 1951;73(6):2450–2. doi:10.1021/ja01150a012
Klinkova LA, Nikolaichik VI, Barkovskii NV, Fedotov VK. New phases in the barium-rich region of the BaO–BaCuO2 system. Bull Russ Acad Sci: Phys. 2009;73(8):1104–6. doi:10.3103/S1062873809080243
Zhang W, Osamura K, Ochiai S. Phase diagram of the BaO–CuO binary system. J Am Ceram Soc. 1990;73(7):1958–64. doi:10.1111/j.1151-2916.1990.tb05252.x
http://www.ihte.uran.ru/?page_id=3142
DOI: https://doi.org/10.15826/chimtech.2019.6.2.01
Copyright (c) 2019 Gennady Vdovin, Anna Rudenko, Boris Antonov, Vacheslav Malkov, Anatoly Demin, Dmitry Medvedev

This work is licensed under a Creative Commons Attribution 4.0 International License.
Chimica Techno Acta, 2014–2025
eISSN 2411-1414
Copyright Notice







