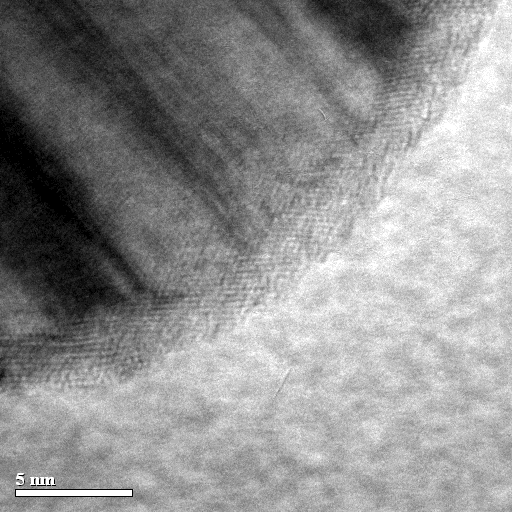
Encapsulation of Ni nanoparticles with oxide shell in vapor condensation
Abstract
Keywords
Full Text:
PDFReferences
Gusev AI. Nanomaterialy, nanostructury, nanotekhnologii. Moscow: Fizmatlit, 2005. 416 p. Russian.
Kotov YuA, Rhee ChK, Beketov IV, Bagazeyev AV, Demina TM, Murzakayev AM, Samatov OM, Timoshenkova OR, Medvedev AI, Shtols AK. Production of Copper Nanopowders by Electric Explosion of Wire-Study of Their Oxidation during Storage and Heating in Air. Journal of Metastable and Nanocrystalline Materials. 2003;15-16:343-8. doi:10.4028/www.scientific.net/JMNM.15-16.343
Athanassiou EK, Grass RN, Stark WJ. Large-scale production of carbon-coated copper nanoparticles for sensor applications. Nanotechnology. 2006;17(6):1668-73. doi:10.1088/0957-4484/17/6/022
Hayashi C. Ultrafine particles. Journal of Vacuum Science & Technology A. 1987;5(4):1375-84. doi:10.1116/1.574773
Tsang SC, Chen YK, Harris PJF, Green MLH. A simple chemical method of opening and filling carbon nanotubes. Nature. 1994;372(6502):159-62. doi:10.1038/372159a0
Tomita S, Hikita M, Fujii M, Hayashi S, Akamatsu K, Deki S, Yasuda H. Formation of Co filled carbon nanocapsules by metal-template graphitization of diamond nanoparticles. Journal of Applied Physics. 2000;88(9):5452-6. doi:10.1063/1.1317242
Zhang ZD, Zheng JG, Skorvanek I, Wen GH, Kovac J, Wang FW, Yu JL, Li ZJ, Dong XL, Jin SR, Liu W, Zhang XX. Shell/core structure and magnetic properties of carbon-coated Fe-Co(C) nanocapsules. Journal of Physics: Condensed Matter. 2001;13(9):1921-9. doi:10.1088/0953-8984/13/9/314
Wartenberg HV, Reusch HJ, Saran E. Schmelzpunktsdiagramme höchstfeuerfester Oxyde. VII. Systeme mit CaO und BeO. Zeitschrift für anorganische und allgemeine Chemie. 1937;230(3):257-76. German. doi:10.1002/zaac.19372300309
Evans UR. Corrosion and Oxidation of Metals. London: Edward Arnold Ltd., 1960. 324 p.
Kotov YA. Electric Explosion of Wires as a Method for Preparation of Nanopowders. Journal of Nanoparticle Research. 2003;5(5):539-50. doi:10.1023/B:NANO.0000006069.45073.0b
Pohil PF, Belyaev AF et al. Gorenie poroshkoobraznykh metallov v aktyivnykh sredakh. Moscow: Nauka, 1972. 294 p. Russian.
Payne BP, Biesinger MC, McIntyre NS. The study of polycrystalline nickel metal oxidation by water vapour. Journal of Electron Spectroscopy and Related Phenomena. 2009;175(1):55-65. doi:10.1016/j.elspec.2009.07.006
Weidler N, Schuch J, Knaus F, Stenner P, Hoch S, Maljusch A, Schäfer R, Kaiser B, Jaegermann W. X-ray Photoelectron Spectroscopic Investigation of Plasma-Enhanced Chemical Vapor Deposited NiOx, NiOx(OH)y, and CoNiOx(OH)y: Influence of the Chemical Composition on the Catalytic Activity for the Oxygen Evolution Reaction. Journal of Physical Chemistry C. 2017;121(12):6455-63. doi:10.1021/acs.jpcc.6b12652
Leedahl B, Boukhvalov DW, Kurmaev EZ, Kukharenko A, Zhidkov IS, Gavrilov NV, Cholakh SO, Huu Le P, Wei Luo C, Moewes A. Bulk vs. Surface Structure of 3d Metal Impurities in Topological Insulator Bi2Te3. Scientific Reports. 2017;7(1):5758. doi:10.1038/s41598-017-06069-3
Perel’man FM, Zvorykin AYa. Kobalt i nikel. Мoscow: Nauka, 1975. 215 p. Russian.
DOI: https://doi.org/10.15826/chimtech.2019.6.3.02
Copyright (c) 2019 Beketov IV, SafronovAP, Medvedev AI, Murzakaev AM, Zhidkov IS, Cholah SO, Maximov AD

This work is licensed under a Creative Commons Attribution 4.0 International License.
Chimica Techno Acta, 2014–2025
eISSN 2411-1414
Copyright Notice






