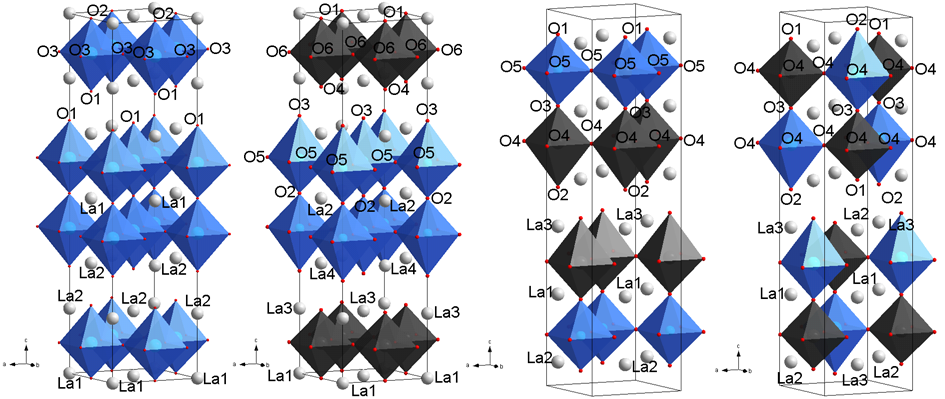
Investigations into the structure of La3Ni2−xFexO7±δ
Abstract
Keywords
Full Text:
PDFReferences
Amow G, Davidson IJ, Skinner SJ. A comparative study of the Ruddlesden-Popper series, Lan+1NinO3n+1 (n = 1, 2 and 3), for solid-oxide fuel-cell cathode applications. Solid State Ionics. 2006;177(13–14):1205–10. doi:10.1016/j.ssi.2006.05.005
Goettler R, Xing Z, Xue L, Hill M. Evaluation of Ruddlesden-Popper nickelate cathodes for higher temperature SOFC. Ceramic Engineering and Science Proceedings. 2008;28(4):183–94. doi: 10.1002/9780470339534.ch18
Takahashi S, Nishimoto S, Matsuda M, Miyake M, Electrode properties of the Ruddlesden–Popper series, Lan+1NinO3n+1 (n= 1, 2, and 3), as intermediate-temperature solid oxide fuel cells. J. Am. Ceram. Soc. 2010;93(8):2329-33. doi: 10.1111/j.1551-2916.2010.03743.x
Woolley RJ, Skinner SJ. Novel La2NiO4+δ and La4Ni3O10−δ composites for solid oxide fuel cell cathodes. J. Power Sources. 2013;243:790–95. doi: 10.1016/j.jpowsour.2013.06.106
Woolley RJ, Skinner SJ. Functionally graded composite La2NiO4+δ and La4Ni3O10−δ solid oxide fuel cell cathodes. Solid State Ionics. 2014;255:1–5. doi: 10.1016/j.ssi.2013.11.041
Brisi C., Vallino M, Abbattista F. Composition and structure of two hitherto unidentified phases in the system La2O3-NiO-O. J. Less Common Metals, 1981;79(2): 215-19. doi: 10.1016/0022-5088(81)90070-9
Drennan J, Tavares CP, Steele BCH. An electron microscope investigation of phases in the system La–Ni–O. Mat. Res. Bull. 1981;17(5):621-26. doi: 10.1016/0025-5408(82)90044-7
Odier P, Nigara Y, Coutures J, Sayer M. Phase relations in the La–Ni–O system: Influence of temperature and stoichiometry on the structure of La2NiO4. J. Solid State Chem. 1985;56(1):32-40. doi: 10.1016/0022-4596(85)90249-X
Ram RM, Ganapathi L, Ganguly P, Rao CNR. Evolution of three-dimensional character across the Lan+1NinO3n+1 homologous series with increase in n. J. Solid State Chem. 1986;63(2):139-47. doi: 10.1016/0022-4596(86)90163-5
Sreedhar K, McElfresh M, Perry D, Kim D, Metcalf P, Honig JM. Low-temperature electronic properties of the Lan+1NinO3n+1 (n= 2, 3, and∞) system: evidence for a crossover from fluctuating-valence to Fermi-liquid-like behavior. J. Solid State Chem. 1994;110(2):208-15. doi: 10.1006/jssc.1994.1161
Zhang Z, Greenblatt M, Goodenough JB. Synthesis, structure, and properties of the layered perovskite La3Ni2O7-δ. J. Solid State Chem. 1994;108(2):402-09. doi: 10.1006/jssc.1994.1059
Carvalho MD, Costa FMA, Pereira IS, Wattiaux A, Bassat JM, Grenier JC, Pouchard M. New preparation method of Lan+1NinO3n+1–δ (n= 2, 3). J. Mater. Chem. 1997;7(10):2107-11. doi: 10.1039/A702424J
Sasaki H, Harashina H, Taniguchi S, Kasai M, Kobayashi Y, Sato M, Sakata M. Structural studies on the phase transition of La3Ni2O6.92 at about 550, K. J. Phys. Soc. Jpn. 1997;66(6):1693-97. doi: 10.1143/jpsj.66.1693
Ling CD, Argyriou DN, Wu G, Neumeier JJ. Neutron diffraction study of La3Ni2O7: Structural relationships among n= 1, 2, and 3 phases Lan+1NinO3n+1. J. Solid State Chem. 2000;152(2):517-25. doi: 10.1006/jssc.2000.8721
Voronin VI, Berger IF, Cherepanov VA, Gavrilova LY, Petrov AN, Ancharov AI, Nikitenko SG. Neutron diffraction, synchrotron radiation and EXAFS spectroscopy study of crystal structure peculiarities of the lanthanum nickelates Lan+1NinOy (n= 1, 2, 3). Nucl. Instrum. Methods Phys. Res., Sect. A. 2001;470(1-2):202-209. doi: 10.1016/S0168-9002(01)01036-1
Bannikov DO, Cherepanov VA. Thermodynamic properties of complex oxides in the La–Ni–O system. J. Solid State Chem. 2006;179(8):2721-27. doi: 10.1016/j.jssc.2006.05.026
Savchenko VF, Ivashkevich LS,. Lubkina IYa, Russ. J. Inorg. Chem. 1988;33:30-33.
Kobayashi Y, Taniguchi S, Kasai M, Sato M, Nishioka, Kontani M. Transport and magnetic properties of La3Ni2O7-δ and La4Ni3O10-δ. J. Phys. Soc. Jpn. 1996;65(12):3978-82. doi: doi.org/10.1143/jpsj.65.3978
Gervais F, Odier P, Nigara Y. Plasmon behavior at the “semiconductor-metal” transition in La2NiO4 and La3Ni2O7. Solid State Commun. 1985;56(4):371-74. doi: 10.1016/0038-1098(85)90405-3
Zinkevich M, Solak N, Nitsche H, Ahrens M, Aldinger F. Stability and thermodynamic functions of lanthanum nickelates. J. Alloys Compd. 2007; 438(1-2):92-99. doi: 10.1016/j.jallcom.2006.08.047
Fontcuberta J, Longworth G, Goodenough JB. Magnetic order or charge-density wave in La2NiO4 by Mössbauer spectroscopy. Phys. Rev. B. 1984;30(11):6320. doi: 10.1103/PhysRevB.30.6320
Tsipis EV, Naumovich EN, Patrakeev MV, Waerenborgh JC, Pivak YV, Gaczyński P, Kharton VV. Oxygen non-stoichiometry and defect thermodynamics in La2Ni0.9Fe0.1O4+δ. J. Phys. Chem. Solids. 2007;68(7):1443-55. doi: 10.1016/j.jpcs.2007.04.006
Klande T, Efimov K, Cusenza S, Becker KD, Feldhoff A. Effect of doping, microstructure, and CO2 on La2NiO4+δ-based oxygen-transporting materials. J. Solid State Chem. 2011;184(12):3310-18. doi: 10.1016/j.jssc.2011.10.019
Klande T, Cusenza S, Gaczyński P, Becker KD, Dörrer L, Borchardt G, Feldhoff A. In-situ Mössbauer studies of 57Fe-doped Ruddlesden–Popper type lanthanum nickel oxides. Solid State Ionics, 2012;222-223:8-15. doi: 10.1016/j.ssi.2012.06.019
Carvalho MD, Wattiaux A, Ferreira LP, Bassat JM. Mössbauer investigation of 57Fe doped La4Ni3O10±y phases. J. Solid State Chem. 2009;182(1):60-64. doi: 10.1016/j.jssc.2008.10.006
Tsipis EV, Patrakeev MV, Waerenborgh JC, Pivak YV, Markov AA, Gaczyński P, Kharton VV. Oxygen non-stoichiometry of Ln4Ni2.7Fe0.3O10−δ (Ln= La, Pr). J. Solid State Chem. 2007;180(6):1902-10. doi: 10.1016/j.jssc.2007.04.025
Rodriguez-Carvajal, J. Commission on powder diffraction (IUCr). Newsletter, 2001;26:12.
Lagarec K, Rancourt DG. RECOIL, Mössbauer spectral analysis software for windows (version 1.02). Ottawa :Department of Physics, University of Ottawa;1998.
Gütlich P, Bill E, Trautwein AX, Mössbauer Spectroscopy and Transition Metal Chemistry. Berlin, Heidelberg: Springer; 2011. 568 p. doi: 10.1007/978-3-540-88428-6
Menil F. Systematic trends of the 57Fe Mössbauer isomer shifts in (FeOn) and (FeFn) polyhedra. Evidence of a new correlation between the isomer shift and the inductive effect of the competing bond TX (→ Fe)(where X is O or F and T any element with a formal positive charge). J. Phys. Chem. Solids. 1985;46(7):763-89. doi: 10.1016/0022-3697(85)90001-0
Rancourt DG. Accurate site populations from Mössbauer spectroscopy. Nucl. Instrum. Methods Phys. Res., Sect. B. 1989;44(2):199-210. doi: 10.1016/0168-583X(89)90428-X
Boultif A, Louër D. Powder pattern indexing with the dichotomy method. J. Appl. Crystallogr. 2004;37(5):724-31 doi: 10.1107/S0021889804014876
Visser JW. A fully automatic program for finding the unit cell from powder data. J. Appl. Crystallogr. 1969;2(3):89-95. doi: 10.1107/S0021889869006649
Roisnel T, Rodríquez-Carvajal J. WinPLOTR: a windows tool for powder diffraction pattern analysis. In Materials Science Forum (Vol. 378, No. 1, pp. 118-123). Transtec Publications; 1999.
Hahn Th. International Tables for Crystallography, Vol. A: Space-group symmetry. 5th ed. Dordrecht: Springer; 2005. 911 p.
Kraus W, Nolze G. POWDER CELL–a program for the representation and manipulation of crystal structures and calculation of the resulting X-ray powder patterns. J. Appl. Crystallogr. 1996;29(3):301-03. doi: 10.1107/S0021889895014920
Shannon RD. Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Acta Cryst. 1976;A32:751–67. doi: 10.1107/S0567739476001551
Taniguchi S, Nishikawa T, Yasui Y, Kobayashi Y, Takeda J, Shamoto SI, Sato M. Transport, magnetic and thermal properties of La3Ni2O7-δ. J. Phys. Soc. Jpn. 1995;64(5):1644-50. doi: doi.org/10.1143/jpsj.64.1644
Eckold G. UNISOFT – A Program Package for Lattice-Dynamical Calculations: User Manual, 2nd rev. ed. Jülich: Jülich Forschungszentrum;1992.
Stevens JG, Dunlap BD. Nuclear moments and moment ratios as determined by Mössbauer spectroscopy. J. Phys. Chem. Ref. Data, 1976;5(4):1093-122. doi: 10.1063/1.555541
Liverts EZ, Zhetbaev AK. Sternheimer Quadrupole Factors in Ferric Compounds. Phys. Status Solidi B. 1982;111(2):469-475. doi: 10.1002/pssb.2221110207
DOI: https://doi.org/10.15826/chimtech.2019.6.2.03
Copyright (c) 2019 Evgeny Alexandrovich Kiselev, Piotr Gaczynski, Götz Eckold, Armin Feldhoff, Klaus-Dieter Becker, Vladimir Alexandrovich Cherepanov

This work is licensed under a Creative Commons Attribution 4.0 International License.
Chimica Techno Acta, 2014–2025
eISSN 2411-1414
Copyright Notice






