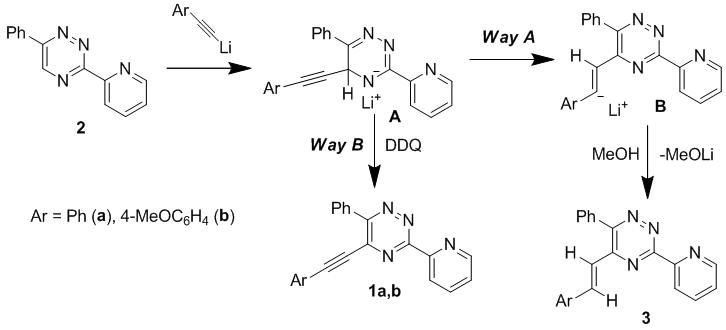
Direct synthesis of 5-arylethynyl-1,2,4-triazines via direct CH-functionalization
Abstract
Keywords
Full Text:
PDFReferences
(a) Heravi MM, Sadjadi S. Recent advances in the application of the Sonogashira method in the synthesis of heterocyclic compounds. Tetrahedron. 2009;65:7761-7775. doi:10.1016/j.tet.2009.06.028 ; (b) Verma AK, Jha RR, Chaudhary R, Tiwari RK, Reddy KSK, Danodia A. Copper-catalyzed tandem synthesis of indolo-, pyrrolo[2,1-a]isoquinolines, naphthyridines and bisindolo/pyrrolo[2,1-a]isoquinolines via hydroamination of ortho-haloarylalkynes followed by C-2 arylation. J Org Chem. 2012;77:8191-8205. doi:10.1021/jo301572p
(a) Pasini D. The Click Reaction as an Efficient Tool for the Construction of Macrocyclic Structures. Molecules. 2013;18:9512-9530. doi:10.3390/molecules18089512 ; (b) Hiroki H, Ogata K, Fukuzawa S-I. 2-Ethynylpyridine-promoted rapid copper(i) chloride catalyzed azide–alkyne cycloaddition reaction in water. Synlett. 2013;24:843-846. doi:10.1055/s-0032-1318488 ; (c) Kovács S, Zih-Peréni K, Révész Á, Novák Z. Copper on iron: catalyst and scavenger for azide–alkyne cycloaddition. Synthesis. 2012;44:3722-3730. doi:10.1055/s-0032-1317697
(a) Benniston AC, Harriman A, Lawrie DJ, Mayeux A, Rafferty K, Russell OD. A general purpose reporter for cations: absorption, fluorescence and electrochemical sensing of zinc(ii). Dalton Trans. 2003;4762-4769. doi:10.1039/B313413J ; (b) Joshi HS, Jamshidi R, Tor Y. Conjugated 1,10-Phenanthrolines as Tunable Fluorophores. Angew Chem Int Ed. 1999;38:2722-2725. doi:10.1002/(SICI)1521-3773(19990917)38:18<2721::AID-ANIE2721>3.0.CO;2-5
John R. Carson, inventor; McNeilab, Inc., assignee. Heteroaromatic acetylenes useful as anthypertensive agents. US Patent 4,663,334. 1987.
Kozhevnikov DN, Kozhevnikov VN, Prokhorov AM, Ustinova MM, Rusinov VL, Chupakhin ON, Aleksandrov GG, König B. Consecutive nucleophilic substitution and aza Diels–Alder reaction—an efficient strategy to functionalized 2,2′-bipyridines. Tetrahedron Lett. 2006;47(6): 869-872. doi:10.1016/j.tetlet.2005.12.006
Kopchuk DS, Nikonov IL, Khasanov AF, Giri K, Santra S, Kovalev IS, Nosova EV, Gundala S, Venkatapuram P, Zyryanov GV, Majee A, Chupakhin ON. Studies on the interactions of 5-R-3-(2-pyridyl)-1,2,4-triazines with arynes: inverse demand aza-Diels–Alder reaction versus aryne-mediated domino process. Org Biomol Chem. 2018;16:5119-5135. doi:10.1039/C8OB00847G
Kopchuk DS, Krinochkin AP, Khasanov AF, Kovalev IS, Slepukhin PA, Starnovskaya ES, Mukherjee A, Rahman M, Zyryanov GV, Majee A, Rusinov VL, Chupakhin ON, Santra S. An Efficient Cyanide-Free Approach towards 1-(2-Pyridyl)isoquinoline-3-carbonitriles via the Reaction of 5-Phenacyl-1,2,4-triazines with 1,2-Dehydrobenzene in the Presence of Alkyl Nitrites. Synlett. 2018;29:483-488. doi:10.1055/s-0036-1590961
Prokhorov AM, Slepukhin PA, Kozhevnikov DN. CuCl2 induced reactions of 6-ethynyl- and 6-cyano-5-aryl-2,2’-bipyridines with various N- and O-nucleophiles in comparison with the reactions of relative 1,2,4-triazines. J Organometallic Chem. 2008;693:1886-1894. doi:10.1016/j.jorganchem.2008.02.016
Kopchuk DS, Nikonov IL, Krinochkin AP, Kovalev IS, Zyryanov GV, Rusinov VL, Chupakhin ON. One-pot non-cyanide synthesis of 1-(pyridin-2-yl)isoquinoline-3-carbonitrile by reaction of 1-phenyl-2-[6-phenyl-3-(pyridin-2-yl)-1,2,4-triazin-5-yl]ethanone with 1,2-dehydrobenzene in the presence of isoamyl nitrite. Russ J Org Chem. 2017;53(6):959-961. doi:10.1134/S1070428017060264
(a) Krinochkin AP, Kopchuk DS, Kovalev IS, Zyryanov GV, Rusinov VL, Chupakhin ON. One-Step synthesis of 5-methyl-1,2,4-triazines by the transformation of their 5-phenacyl derivatives. Russ J Org Chem. 2019;55(2):266-268. doi:10.1134/S1070428019020210; (b) Krinochkin AP, Kopchuk DS, Kim GA, Shevyrin VA, Santra S, Rahman M, Taniya OS, Zyryanov GV, Rusinov VL, Chupakhin ON. Water-soluble luminescent lanthanide complexes based on C6-DTTA-appended 5-aryl-2,2′-bipyridines. Polyhedron. 2020;181:114473. doi 10.1016/j.poly.2020.114473
Shoetsu K, Satoshi F, Hiroshi Y. Studies on as-Triazine Derivatives. V. Synthesis and Hydration of Alkynyl-1,2,4-triazines. Heterocycles. 1984;22(10):2245-2248.
Prokhorov AM, Makosza M, Chupakhin ON. Direct introduction of acetylene moieties into azines by SNH methodology. Tetrahedron Lett. 2009;50(13):1444-1446. doi:10.1016/j.tetlet.2009.01.070
Carroll FI, Kotturi SV, Navarro HA, Mascarella SW, Gilmour BP, Smith FL, Gabra BH, Dewey WL. Synthesis and pharmacological evaluation of phenylethynyl[1,2,4]methyltriazines as analogues of 3-methyl-6-(phenylethynyl)pyridine. J Med Chem. 2007;50(14):3388-3391. doi:10.1021/jm070078r
Khasanov AF, Kopchuk DS, Kovalev IS, Taniya OS, Zyryanov GV, Rusinov VL, Chupakhin ON. Reaction of lithium 2-arylethynides with 6-aryl-3-(2-pyridyl)-1,2,4-triazines as an access to 6-aryl-5-arylvinyl-3-(2-pyridyl)-1,2,4-triazines. Mendeleev Commun. 2015;25(5):332-333. doi:10.1016/j.mencom.2015.09.003
Kozhevnikov VN, Shabunina OV, Kopchuk DS, Ustinova MM, König B, Kozhevnikov DN. Facile synthesis of 6-aryl-3-pyridyl-1,2,4-triazines as a key step towards highly fluorescent 5-substituted bipyridines and their Zn(II) and Ru(II) complexes. Tetrahedron. 2008;64:8963-8973. doi:10.1016/j.tet.2008.06.040
DOI: https://doi.org/10.15826/chimtech.2020.7.3.02
Copyright (c) 2020 Savchuk MI, Starnovskaya ES, Shtaitz YK, Krinochkin AP, Kopchuk DS, Santra S, Rahman M, Zyryanov GV, Rusinov VL, Chupakhin ON

This work is licensed under a Creative Commons Attribution 4.0 International License.
Chimica Techno Acta, 2014–2025
eISSN 2411-1414
Copyright Notice






