New pathways for the synthesis of indolyl-containing quinazoline trifluoroacetohydrazides
Abstract
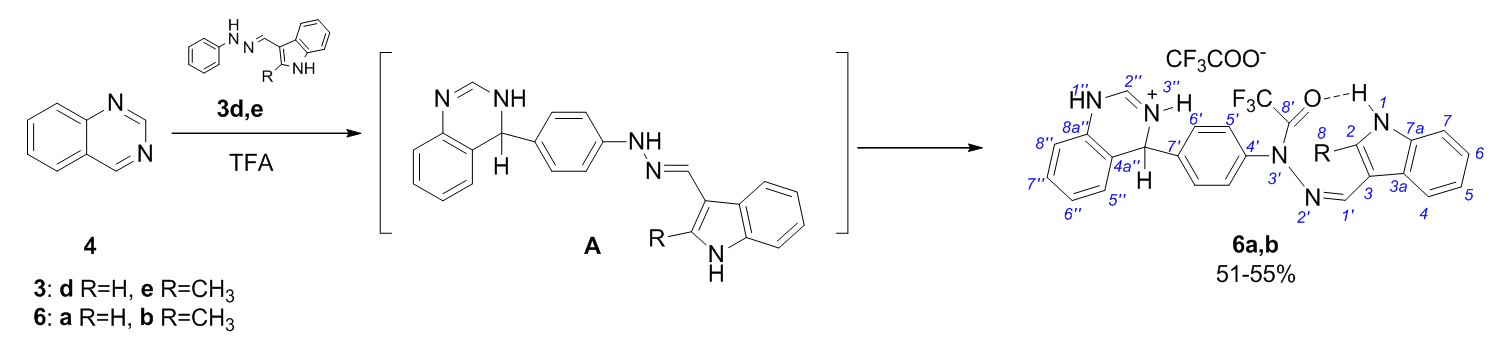
The reactions of indole-3-carbaldehyde arylhydrazones with quinazoline in TFA proceed at the 7' position of the aryl part of the hydrazone molecule to form σ-adducts of quinazoline trifluoroacetohydrazides.
Keywords
Full Text:
PDFReferences
D’yakonov AL, Telezhenetskaya MV. Quinazoline alkaloids in nature. Chem. Nat. Compd. 1997;33:221-267. doi:10.1007/BF02234869
Aniszewski T. Alkaloids (Second Edition). Helsinki: Elsevier Science, 2015. 496 p.
Asif M. Chemical Characteristics, Synthetic Methods, and Biological Potential of Quinazoline and Quinazolinone Derivatives. Int. J. Med. Chem. 2014;ID 395637:1-27. doi:10.1155/2014/395637
Solyanik GI. Quinazoline compounds for antitumor treatment. Exp. Oncology. 2019;41:3-6. doi:10.32471/exp-oncology.2312-8852.vol-41-no-1.12414
Tregubenko IP, Tarakhtii EA, Chibiriak MV, Golomolzin BV, Egorova LG. K voprosu o mekhanizme deystviya dialkilaminoetiltiol'nykh proizvodnykh pirimidina i khinazolina. Radiobiologiia. 1984;24:838-846. Russian. Available from: https://elibrary.ru/item.asp?id=25627353
Pilicheva TL, Chupakhin ON, Postovsky IYa. Addition of nucleophiles to 3-methylquinazolinium iodide. Chem. Het. Comp. 1975;11:496-499. doi:10.1007/BF00502444
Azev YuA, Shorshnev SV, Golomolzin BV. Specific features of the reactions of quinazoline and its 4-hydroxy and 4-chloro substituted derivatives with C-nucleophiles. Tetr. Lett. 2009;50:2899-2903. doi:10.1016/j.tetlet.2009.03.199
Azev YuA, Koptyaeva OS, Seliverstova EA, Ivoilova AV, Pospelova TA. Synthesis of Stable σ-Adducts by Arylation of Quinazoline. Rus. J. Gen. Chem. 2019;89(12):2374-2377. doi:10.1134/S1070363219120089
Sundberg RJ. Indoles. San Diego: Academic Press Inc, 1996. 175 p.
Chadra N, Silakari O. Key Heterocycle Cores for Designing multitargeting molecules. Patiala: Elsevier, 2018, 285 p. doi:10.1016/B978-0-08-102083-8.00008-X
Anastas PT, Warner JC. Green Chemistry Theory and Practice. New York: Oxford University Press, 1998, 516 p.
Charushin VN, Chupakhin ON. Topics in Heterocyclic Chemistry: Metal-free C-H functionalization of aromatic compounds through the action of nucleophilic reagents. Eds.: Charushin VN, Chupakhin ON. Switzerland: Springer; 2014;37:1-50.
Makosza M, Wojciechowski K. Topics in Heterocyclic Chemistry: Nucleophilic Substitution of Hydrogen in Arenes and Heteroarenes. Eds.: Charushin VN, Chupakhin ON. Switzerland: Springer; 2014;37:51-105.
Azev YuA, Koptyaeva OS, Eltzov OS, Yakovleva YuA, Pospelova TA, Bakulev VA. Quinazoline addition to indole hydrazone derivatives in TFA as a facile synthesis of trifluoroacetylhydrazide quinazoline σ-adducts. Mend. Comm. 2020;30:226-227. doi:10.1016/j.mencom.2020.03.032
Zabaleta N, Uria U, Reyes E, Carrillo L, Vicario JL. Ion-pairing catalysis in the enantioselective addition of hydrazones to N-acyldihydropyrrole derivatives. Chem. Comm. 2018;54:8905-8908. doi:10.1039/C8CC05311A
Tung T, Tezcan H, San M, Bueykguengoer O, Yagbasan R. N-(4-Nitrobenzylidene)-N′-phenylhydrazine. Acta Cryst., Sec. C. 2003;59:528-529. doi:10.1107/S0108270103016019
DOI: https://doi.org/10.15826/chimtech.2020.7.3.03
Copyright (c) 2020 Yurii Azev, Olga Koptyaeva, Oleg Eltsov, Yuliya Yakovleva, Tatyana Pospelova

This work is licensed under a Creative Commons Attribution 4.0 International License.
Chimica Techno Acta, 2014–2025
eISSN 2411-1414
Copyright Notice






