To the question of the influence of a silanol cover on the protolytic properties of aminopropyl silica gels
Abstract
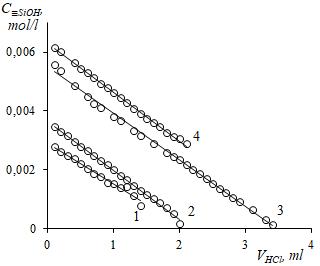
The paper proposes a model that describes the acid-base properties of amino groups grafted onto the surface, taking into account their interaction with silanol groups. For this, aminopropyl silica was chosen as an object with well-studied methods of preparation, structure and properties. The isotherms of sorption of hydrogen ions on aminopropyl silica were obtained by potentiometric method. The experimental points were analyzed numerically, taking into account the presence of an electric double layer, the presence of surface processes competing with the sorption of hydrogen ions, and the peculiarities of the behavior of silanol groups when the degree of surface filling with hydrogen ions changes. The resulting model makes it possible to carry out a preliminary calculation of the sorption of hydrogen ions on the surface of aminopropyl silica.
Keywords
Full Text:
PDFReferences
Davis GA, Kent DB. Surface complexation models in aqueous geochemistry. Rev. Mineral. Geochem. 1990;23(1):177–260.
Davis JA, James RO, Leckie JO. Surface ionization and complexation at the oxide / water interface. I. Computation of electrical double layer properties in simple electrolytes. J. Colloid Interface Sci. 1978;63(3):480–99. doi:10.1016/S0021-9797(78)80009-5
Kropacheva TN, Didik MV, Kornev VI. Modelirovaniye sorbtsii kationov tyazhelykh metallov gidroksidami v prisutstvii EDTA [Modeling of the heavy metal cations sorption by hydroxides in the EDTA presence]. Sorbtsionnyye i khromatograficheskiye protsessy. 2013;13(3):360–68. Russian.
Demianenko EM, Vlasova NN, Golovkova LP, Grebenyuk AG, Kuts VS, Lobanov VV. Izucheniye adsorbtsii akridina i proflavina na poverkhnosti kremnezema [Study of Acridine and Proflavine Adsorption on Silica Surface]. Khimiya, fizika i tekhnologiya poverkhnosti. 2012;3(2):142–54. Russian.
Moira KR, Machesky ML, Kubicki JD. Anatase Nanoparticle Surface Reactivity in NaCl Media: A CD – MUSIC Model Interpretation of Combined Experimental and Density Functional Theory Studies. Langmuir. 2013;29(27):8572–83. doi:10.1021/la4011955
Kholin YuV. Kolichestvennyy fiziko-khimicheskiy analiz kompleksoobrazovaniya v rastvorakh i na poverkhnosti khimicheski modifitsirovannykh kremnezemov: soderzhatel'nyye modeli, matematicheskiye metody. Khar'kov: Folio; 2000. 290 p. Russian.
Khristenko IV, Kholin YuV. The greement of the results of quantitative physical-chemical analysis and probing surfaces of aminosilicas by Reichardt’s solvatochromic betaine indicators. Kharkov University Bulletin. 2007;13(38):245-50.
Kobylinskaya NG, Zaytsev VN. Izucheniye protoliticheskikh svoystv kremnezemov, modifitsirovannykh 8-aminometilkhinolinom [Study of the modified with 8-aminomethylquinoline silicas protolytic properties]. Trudy Odesskogo politekhnicheskogo universiteta. 2006;1(25):231–6. Russian.
Barkauskas J, Dervinyte M. An investigation of the functional groups on the surface of activated carbons. J. Serb. Chem. Soc. 2004;69(5):363–75. doi:10.2298/JSC0405363B
Chen L.C., Peng P.Y., Lin L.F., Yang T.C., Huang C.M. Facile preparation of nitrogen-doped activated carbon for carbon dioxide adsorption Aerosol Air Qual. Res. 2014;14(3):916–27. doi:10.4209/aaqr.2013.03.0089
Zaytsev VN. Funktsionalizirovannyye materialy. Tom 1. Kompleksoobrazuyushchiye kremnezemy: sintez, stroyeniye privitogo sloya i khimiya poverkhnosti. Seriya monogr. pod red. akad. V.V. Skopenko [Functionalized materials. Volume 1. Complexing silicas: synthesis, graft structure and surface chemistry. Monogr. ed. acad. V.V. Skopenko]. Khar'kov: Folio; 1997. 240 p. Russian.
Zhu M, Lerum MZ, Chen W. How to prepare reproducible, homogeneous, and hydrolytically stable aminosilane-derived layers on silica. Langmuir. 2012;28(1):416–23. doi:10.1021/la203638g
Bol'bukh YuN. Vysokodispersnyye kremnezemy s binarnymi aminometil'nymi i kremniygidrometil'nymi modifitsiruyushchimi sloyami [Highly dispersed silicas with the binary aminomethyl and silicon-hydromethyl modifying layers]. Nanostrukturnoye materialovedeniye. 2011;(2):44–61. Russian.
Sharov AV, Morozova TV, Kovyatkin YaV, Filisteev OV. Kolichestvennoye opisaniye vliyaniya silanol'nogo pokrova na kislotno-osnovnyye svoystva 3-aminopropilsilikageley [Quantitative description of silanol cover influence on the 3-aminopropyl silica acid-base properties]. Sorbtsionnyye i khromatograficheskiye protsessy. 2015;15(2):243–50. Russian.
Koopal LK, Yang Y, Minnaard AJ, Theunissen PLM, van Riemsdijk WH. Chemical immobilization of humic acid on silica. Colloids Surf. A. 1998;141(3):385–95. doi:10.1016/S0927-7757(97)00170-2
Vlasova NN. Sravneniye modeley kompleksoobrazovaniya na poverkhnosti dlya kolichestvennogo opisaniya kislotnykh svoystv vysokodispersnogo kremnezema [A comparison of surface complexation models for quantitative description of acidic properties of fumed silica]. Khimiya, fizika i tekhnologiya poverkhnosti. 2008;14:6–15. Russian.
Nikolsky BP, editor. Spravochnik khimika [Chemist’s handbook]. Vol 3. Leningrad (USSR): Khimiya; 1965. 1008 p. Russian.
DOI: https://doi.org/10.15826/chimtech.2020.7.4.15
Copyright (c) 2020 O. V. Filisteev, A. V. Sharov

This work is licensed under a Creative Commons Attribution 4.0 International License.
Chimica Techno Acta, 2014–2025
eISSN 2411-1414
Copyright Notice







