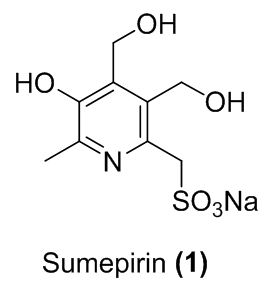
Synthetic route optimization of Sumepirin antiepileptic drug candidate
Abstract
Keywords
Full Text:
PDFReferences
Janmohamed M, Brodie MJ, Kwan P. Pharmacoresistance – Epidemiology, mechanisms, and impact on epilepsy treatment. Neuropharmacology. 2020;168:107790 doi:10.1016/j.neuropharm.2019.107790
Štuhec M. Optimization of antiepileptic drug pharmacotherapy related to adverse drug effects: How to approach . Farm Vestn. 2015;66(1):28–34.
Korytnyk W. A seven-membered cyclic ketal of pyridoxol. J Org Chem. 1962;27(10):3724–6.
Shtyrlin NV, Strel’Nik AD, Sysoeva LP, Lodochnikova OA, Klimovitskii EN, Shtyrlin YG. New synthetic method for 2,3,4-tris(hydroxymethyl)- 6-methylpyridin-5-ol. Russ J Org Chem. 2009;45(8):1266–8. doi:10.1134/S1070428009080314
Pugachev MV, Shtyrlin NV, Sysoeva LP, Nikitina EV, Abdullin TI, Iksanova AG, Ilaeva AA, Berdnikov EA, Musin RZ, Shtyrlin YG. Synthesis and antibacterial activity of novel phosphonium salts on the basis of pyridoxine. Bioorg Med Chem. 2013;21(14):4388–95. doi:10.1016/j.bmc.2013.04.051
Shtyrlin NV, Dobrynin AB, Madzhidov TI, Pugachev MV, Sysoeva LP, Musin RZ, Litvinov IA, Klimovitskii EN, Shtyrlin YG. Experimental and theoretical study on 6-substituted pyridoxine derivatives. Synthesis of Cyclic 2,4,5,6-Tetrakis-(hydroxymethyl)pyridin-3-ol Acetonides. Russ J Org Chem. 2011;47(1):100–8. doi:10.1134/S107042801101012X
Lima CGS, Pauli FP, Costa DCS, de Souza AS, Forezi LSM, Ferreira VF, Da Silva F.C. para-Quinone Methides as Acceptors in 1,6-Nucleophilic Conjugate Addition Reactions for the Synthesis of Structurally Diverse Molecules. Eur J Org Chem. 2020;2020(18):2650–92. doi:10.1002/ejoc.201901796
Mukhopadhyay S, Gharui C, Pan SC. Applications of Bifunctional Organocatalysts on ortho-Quinone Methides. Asian J Org Chem. 2019;8(11):1970–84. doi:10.1002/ajoc.201900466
Loubinoux B, Miazimbakana J, Gerardin P. Reactivity of new precursors of quinone methides. Tetrahedron Lett. 1989;30(15):1939–42. doi:10.1016/S0040-4039(00)99619-9
Sanner MA, Stansberry M, Weigelt C, Michne WF. Quinone methide from 4-hydroxybenzyl alcohol diacetate: (P-acetoxy)benzylation of β-dicarbonyls. Tetrahedron Lett. 1992;33(37):5287–90. doi:10.1016/S0040-4039(00)79074-5
Khaziev R, Shtyrlin N, Pavelyev R, Nigmatullin R, Gabbasova R, Grishaev D, Shtro A, Galochkina A, Nikolaeva Y, Vinogradova T, Manicheva O, Dogonadze M, Gnezdilov O, Sokolovich E, Yablonskiy P, Balakin K, Shtyrlin Y. Synthesis and antimicrobial activity of adamantyl substituted pyridoxine derivatives. Lett Drug Des Discov. 2019;16(12):1360–9. doi:10.2174/1570180816666190911150705
Finley DR, Bell MG, Borel AG, Bloomquist WE, Cohen ML, Heiman ML, Kriauciunas A, Matthews DP, Miles T, Neel DA, Rito CJ, Sall DJ, Shuker AJ, Stephens TW, Tinsley FC, Winter MA, Jesudason CD. Potent benzimidazolone based human β3-adrenergic receptor agonists. Bioorg Med Chem Lett. 2006;16(21):5691–4. doi:10.1016/j.bmcl.2006.08.010
Murai M, Okamoto K, Miki K, Ohe K. Palladium-catalyzed three-component coupling reactions of 2-(cyanomethyl)phenol, aryl halides, and carbon monoxide. Tetrahedron. 2015;71(26):4432–7. doi:10.1016/j.tet.2015.04.049
Yazarians JA, Jiménez BL, Boyce GR. A regioselective etherification of pyridoxine via an ortho-pyridinone methide intermediate. Tetrahedron Lett. 2017;58(23):2258–60. doi:10.1016/j.tetlet.2017.04.082
Shearing EA, Smiles S. Derivatives of o-hydroxybenzylsulphonic acid. J Chem Soc. 1937;1348–51. doi:10.1039/jr9370001348
Jankowski P, Poterała M, Lindahl N, Wieczorek W, Johansson P. Chemically soft solid electrolyte interphase forming additives for lithium-ion batteries. J Mater Chem A. 2018;6(45):22609–18. doi:10.1039/c8ta07936f
DOI: https://doi.org/10.15826/chimtech.2020.7.4.04
Copyright (c) 2020 M. S. Dzyurkevich, N. V. Shtyrlin, Y. G. Shtyrlin

This work is licensed under a Creative Commons Attribution 4.0 International License.
Chimica Techno Acta, 2014–2025
eISSN 2411-1414
Copyright Notice






