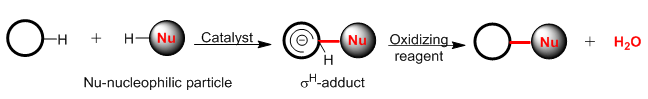
Direct CH/CH functionalization of 1,3-dihydroxy-9H-xanthen-9-one and 1,3-dimethoxy-9H-xanthen-9-one with 1,2,4-triazines and quinazoline
Abstract
An electron-deficient series of 1,2,4-triazines and quinazoline have been used for cross-dehydrogenative coupling with 1,3-dihydroxy and 1,3-dimethoxyxanthones to give stable nucleophilic addition products. The adducts and their subsequent oxidation products were obtained in good yields and the structures of the compounds were confirmed by 1H NMR spectroscopy. These results expand the scope of the methodology of nucleophilic substitution of hydrogen with the participation of xanthones with azines. Moreover, this methodology makes it possible to obtain new organic materials based on xanthones, which have a wide spectrum of biological activity.
Keywords
Full Text:
PDFReferences
Dar A, Shaheen F. Analgesic and antioxidant activity of mangiferin and its derivatives: the structure activity relationship. Biol Pharm Bull. 2005;28:596–600. doi:10.1248/bpb.28.596
Khalymbadzha IA, Fatykhov RF, Chupakhin ON. Functionalization of Aromatic N-Heterocycles via C(sp2)-H/C(sp2)-H CDC Reactions. In: Srivastava A., Jana C. (eds) Heterocycles via Cross Dehydrogenative Coupling. Singapore: Springer Nature: 2019. pp. 35–75. doi:10.1007/978-981-13-9144-6_2
Radhakrishna KM, Prakash N. 1,3-Dihydroxy-9H-xanthones and 1,3-dihydroxy-9H-xanthenes. New methods of synthesis. J Org Chem. 1986;51(5):717–23. doi:10.1021/jo00355a024
Fedunov RG. Electronic structures and population dynamics of excited states of xanthione and its derivatives. J Chem Phys. 2017;494:1–10. doi:10.1016/j.chemphys.2017.07.007
DOI: https://doi.org/10.15826/chimtech.2020.7.4.17
Copyright (c) 2020 A. D. Sharapov, R. F. Fatykhov, I. A. Khalymbadzha, O. N. Chupakhin

This work is licensed under a Creative Commons Attribution 4.0 International License.
Chimica Techno Acta, 2014–2025
eISSN 2411-1414
Copyright Notice






