Electrodialysis concentration of sulfuric acid
Abstract
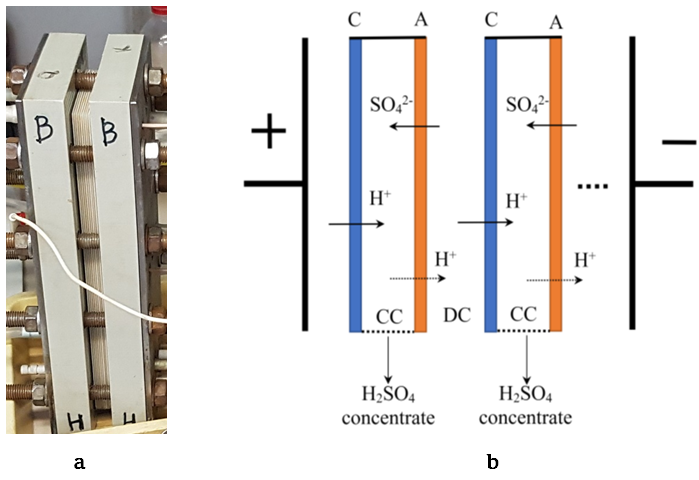
An urgent task is the development of new resource-saving technologies for deep processing of wastes from the hydrometallurgical industry for the purpose of their reuse. Membrane technologies make it possible to create closed technological cycles with the reuse of recovered components in production, which allows solving many environmental problems. At the Abinsk Electric Steel Works Ltd. (Russia), during the production of copper-coated steel wire, a large amount of waste containing sulfuric acid and heavy metal salts is generated. The chemical treatment of such effluents with slaked lime and alkali produces a large amount of sludge, which causes environmental problems and leads to the irreversible loss of sulfuric acid. The problem of separating acids and salts can be solved using diffusion dialysis through anion-exchange membranes, however, to return the acid to the production cycle, the purified acid must be additionally concentrated. In this work, we studied the process of electrodialysis concentration of sulfuric acid using heterogeneous ion-exchange membranes Ralex® CMHPP and Ralex® AMHPP (manufactured by MEGA a.s., Czech Republic) which have a polypropylene reinforcing mesh resistant to acids. The main parameters of the electrodialysis concentration process have been determined – the dependence of the concentration of the regenerated sulfuric acid on the concentration at the inlet to the electrodialysis cell and on the current density, as well as the energy consumption for the process. It is shown that with the help of electrodialysis concentration it is possible to obtain sulfuric acid with a concentration of up to 180 g/L, which makes it possible to return it to the production cycle.
Keywords
Full Text:
PDFReferences
Kurniawan TA, Chan GYS, Lo WH, Babel S. Physico-chemical treatment techniques for wastewater laden with heavy metals. Chem Eng J. 2006;118(1):83-98. doi:10.1016/j.cej.2006.01.015
Gurreri L, Tamburini A, Cipollina A, Micale G. Electrodialysis Applications in Wastewater Treatment for Environmental Protection and Resources Recovery: A Systematic Review on Progress and Perspectives. Membranes. 2020;10(7):146. doi:10.3390/membranes10070146
Bazinet L, Geoffroy TR. Electrodialytic Processes: Market Overview, Membrane Phenomena, Recent Developments and Sustainable Strategies. Membranes. 2020;10(9):221. doi:10.3390/membranes10090221
Panagopoulos A, Haralambous KJ, Loizidou M. Desalination brine disposal methods and treatment technologies - A review. Science of the Total Environment. 2019;693:133545. doi:10.1016/j.scitotenv.2019.07.351
Muhammad Y, Lee W. Zero-liquid discharge (ZLD) technology for resource recovery from wastewater: A review. Science of the Total Environment. 2019;681:551-63. doi:10.1016/j.scitotenv.2019.05.062
Yaroslavtsev AB, Nikonenko VV. Ion-exchange membrane materials: Properties, modification, and practical application. Nanotechnologies in Russia. 2009;4(3):137-59. doi:10.1134/S199507800903001X
Melnikov SS, Mugtamov OA, Zabolotsky VI. Study of electrodialysis concentration process of inorganic acids and salts for the two-stage conversion of salts into acids utilizing bipolar electrodialysis. Sep Purif Technol. 2020;235:1-10. doi:10.1016/j.seppur.2019.116198
Kishida M, Harato T, Tokoro C, Owada S. In situ remediation of bauxite residue by sulfuric acid leaching and bipolar-membrane electrodialysis. Hydrometallurgy. 2017;170:58-67. doi:10.1016/j.hydromet.2016.04.012
Wu X, Zhu H, Liu Y, Chen R, Qian Q, Van der Bruggen B. Cr(III) recovery in form of Na2CrO4 from aqueous solution using improved bipolar membrane electrodialysis. Journal of Membrane Science. 2020;604(118097). doi:10.1016/j.memsci.2020.118097
Heinonen J, Zhao Y, Van der Bruggen B.A process combination of ion exchange and electrodialysis for the recovery and purification of hydroxy acids from secondary sources, Separation and Purification Technology. 2020;240:116642. doi:10.1016/j.seppur.2020.116642
Aydin MI, Yuzer B, Hasancebi B, Selcuk H. Application of electrodialysis membrane process to recovery sulfuric acid and wastewater in the chalcopyrite mining industry. Desalination and Water Treatment. 2019;172:206-11 doi:10.5004/dwt.2019.25051
Handojo L, Wardani AK, Regina D, Bella C, Kresnowatia MTAP, Wenten IG. Electro-membrane processes for organic acid recovery. RSC Adv. 2019;9:7854-69. doi:10.1039/c8ra09227c
Sata T, Sata T, Yang W. Studies on cation-exchange membranes having permselectivity between cations in electrodialysis. Journal of Membrane Science. 2002:206(1-2):31-60. doi:10.1016/S0376-7388(01)00491-4
Ge L, Wu L, Wu B, Wang G, Xu T. Preparation of monovalent cation selective membranes through annealing treatment. Journal of Membrane Science. 2014;459:217-22. doi:10.1016/j.memsci.2014.02.025
Khoiruddin DA, Subagjo IGW. Surface modification of ion-exchange membranes: Methods, characteristics, and performance. Journal of Applied Polymer Science. 2017;134(48):1-13. doi:10.1002/app.45540
Zhang Y, Paepen S, Pinoy L, Meesschaert B, Van Der Bruggen B. Selectrodialysis: Fractionation of divalent ions from monovalent ions in a novel electrodialysis stack. Separation and Purification Technology. 2012;88:191-201. doi:10.1016/j.seppur.2011.12.017
Zabolotskii VI, Lebedev KA, Orel IV. Specific Selectivity of Modified Ion-Exchange Membranes. Russian Journal of Electrochemistry. 2003;39(10):1130-3. doi:10.1023/A:1026187823767
Khan MI, Khraisheh M, Almomani F. Fabrication and characterization of pyridinium functionalized anion exchange membranes for acid recovery. Science of the Total Environment. 2019;686:90–6. doi:10.1016/j.scitotenv.2019.05.481
Gueccia R, Randazzo S, Chillura MD, Cipollina A, Micale G. Experimental investigation and modeling of diffusion dialysis for HCl recovery from waste pickling solution. Journal of Environmental Management. 2019;235:202-12. doi:10.1016/j.jenvman.2019.01.028
Wang K, Zhang Y, Huang J, Liu T, Wang J. Recovery of sulfuric acid from a stone coal acid leaching solution by diffusion dialysis. Hydrometallurgy. 2017;173:9-14. doi:10.1016/j.hydromet.2017.07.005
Regel-Rosocka M. A review on methods of regeneration of spent pickling solutions from steel processing. Journal of Hazardous Materials. 2010;177(1-3):57-69. doi:10.1016/j.jhazmat.2009.12.043
Zhang J, Niu D, Zhang X, Hu S. An economical process to recover sulfuric acid and tetrabutylammonium ions from acidic saline wastewater with organics. Desalination and Water Treatment. 2018;129:149-59. doi:10.5004/dwt.2018.23077
Loza NV, Loza SA, Romanyuk NA, Kononenko NA. Experimental and Theoretical Studies of Electrodialysis of Model Solutions Containing Aniline and Sulfuric Acid. Russ J Electrochem 2019;55:871-7. doi:10.1134/S102319351909009X
Loza NV, Loza SA, Kononenko NA, Magalyanov AV. Ion Transport in sulfuric acid solution through anisotropic composites based on heterogeneous membranes and polyaniline. Pet Chem. 2015;55:724-9. doi:10.1134/S0965544115090054
Shetha B, Nath K. Effect of selected process parameters on the electrodialytic separation and concentration of sulfuric acid using graphite electrodes. Chemical Engineering Communications. 2020;207(3):295-305. doi:10.1080/00986445.2019.1587611
Caputo G, Balog I, Giaconia A, Sau S, Pozio A. Experimental Study for HIx Concentration by Electro-Electrodialysis (EED) Cells in theWater Splitting Sulfur-Iodine Thermochemical Cycle. ChemEngineering. 2019;3(2):50 doi:10.3390/chemengineering3020050
DOI: https://doi.org/10.15826/chimtech.2021.8.1.06
Copyright (c) 2021 Sergey Alexeevich Loza, Nikita Alexandrovich Smyshlyaev, Aleksandr Nikolaevich Korzhov, Nazar Aleksandrovich Romanyuk

This work is licensed under a Creative Commons Attribution 4.0 International License.
Chimica Techno Acta, 2014–2025
eISSN 2411-1414
Copyright Notice







