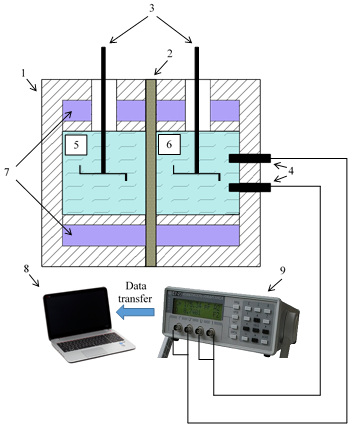
Using a microheterogeneous model to assess the applicability of ion-exchange membranes in the process of reverse electrodialysis
Abstract
Keywords
Full Text:
PDFReferences
Strathmann H. Electrodialysis, a mature technology with a multitude of new applications. Desalination. 2010;264:268–88.
Bazinet L, Geoffroy TR. Electrodialytic processes: Market overview, membrane phenomena, recent developments and sustainable strategies. Membranes (Basel). 2020;10:1–72.
Pärnamäe R, Mareev S, Nikonenko V, Melnikov S, Sheldeshov N, Zabolotskii V, et al. Bipolar membranes: A review on principles, latest developments, and applications. J Memb Sci. 2020;617:118538.
Melnikov SS, Mugtamov OA, Zabolotsky VI. Study of electrodialysis concentration process of inorganic acids and salts for the two-stage conversion of salts into acids utilizing bipolar electrodialysis. Sep Purif Technol. 2020;235:116198.
Loza SA, Smyshlyaev NA, Korzhov AN, Romanyuk NA. Electrodialysis concentration of sulfuric acid. Chimica Techno Acta. 2021;8:20218106.
Bazinet L, Lamarche F, Ippersiel D. Bipolar-membrane electrodialysis: Applications of electrodialysis in the food industry. Trends Food Sci Technol. 1998;9:107–13.
Oren Y, Korngold E, Daltrophe N, Messalem R, Volkman Y, Aronov L, et al. Pilot studies on high recovery BWRO-EDR for near zero liquid discharge approach. Desalination. 2010;261:321–30.
Ahmed FE, Hashaikeh R, Hilal N. Hybrid technologies: The future of energy efficient desalination – A review. Desalination. 2020;495:114659.
Zhao D, Lee LY, Ong SL, Chowdhury P, Siah KB, Ng HY. Electrodialysis reversal for industrial reverse osmosis brine treatment. Sep Purif Technol. 2019;213:339–47.
Tian H, Wang Y, Pei Y, Crittenden JC. Unique applications and improvements of reverse electrodialysis: A review and outlook. Appl Energy. 2020;262:114482.
Mei Y, Tang CY. Recent developments and future perspectives of reverse electrodialysis technology: A review. Desalination. 2018;425:156–74.
Pawlowski S, Huertas RM, Galinha CF, Crespo JG, Velizarov S. On operation of reverse electrodialysis (RED) and membrane capacitive deionisation (MCDI) with natural saline streams: A critical review. Desalination. 2020;476:114183.
Pattle RE. Production of Electric Power by mixing Fresh and Salt Water in the Hydroelectric Pile. Nature. 1954;174:660–660.
Hong JG, Zhang B, Glabman S, Uzal N, Dou X, Zhang H, et al. Potential ion exchange membranes and system performance in reverse electrodialysis for power generation: A review. J Memb Sci. 2015;486:71–88.
Długołecki P, Nymeijer K, Metz S, Wessling M. Current status of ion exchange membranes for power generation from salinity gradients. J Memb Sci. 2008;319:214–22.
Ran J, Wu L, He Y, Yang Z, Wang Y, Jiang C, et al. Ion exchange membranes: New developments and applications. J Memb Sci. 2017;522:267–91.
Pawlowski S, Crespo J, Velizarov S. Sustainable Power Generation from Salinity Gradient Energy by Reverse Electrodialysis. In: Ribeiro AB, editor. Electrokinetics Across Disciplines and Continents. 2016. page 57–80.
Geise GM, Cassady HJ, Paul DR, Logan BE, Hickner MA. Specific ion effects on membrane potential and the permselectivity of ion exchange membranes. Phys Chem Chem Phys. 2014;16:21673–81.
Larchet C, Dammak L, Auclair B, Parchikov S, Nikonenko V. A simplified procedure for ion-exchange membrane characterisation. New J Chem. 2004;28:1260–7.
Zabolotsky VI, Nikonenko VV. Effect of structural membrane inhomogeneity on transport properties. J Memb Sci. 1993;79:181–98.
Akberova EM, Vasil’eva VI, Zabolotsky VI, Novak L. Effect of the sulfocation-exchanger dispersity on the surface morphology, microrelief of heterogeneous membranes and development of electroconvection in intense current modes. J Memb Sci. 2018;566:317–28.
Karpenko L V, Demina OA, Dvorkina GA, Parshikov SB, Larchet C, Auclair B, et al. Comparative Study of Methods Used for the Determination of Electroconductivity of Ion-Exchange Membranes. Russ J Electrochem. 2001;37:287–93.
Kamcev J, Sujanani R, Jang ES, Yan N, Moe N, Paul DR, et al. Salt concentration dependence of ionic conductivity in ion exchange membranes. J Memb Sci. 2017;547:123–33.
Melnikov S, Loza S, Sharafan M, Zabolotskiy V. Electrodialysis treatment of secondary steam condensate obtained during production of ammonium nitrate. Technical and economic analysis. Sep Purif Technol. 2016;157:179–91.
Sarapulova V, Butylskii D, Titorova V, Wang Y, Xu T, Zhang Y, et al. Transport and electrochemical characteristics of CJMCED homogeneous cation exchange membranes in sodium chloride, calcium chloride, and sodium sulfate solutions. Membranes (Basel). 2020;10:1–23.
Sarapulova V, Pismenskaya N, Titorova V, Sharafan M, Wang Y, Xu T, et al. Transport characteristics of CJMAEDTM homogeneous anion exchange membranes in sodium chloride and sodium sulfate solutions. Int J Mol Sci. 2021;22:1–24.
Akberova EM, Vasil’eva VI, Zabolotsky VI, Novak L. A study of ralex membrane morphology by SEM. Membranes (Basel). 2019;9:11–8.
Svoboda M, Beneš J, Vobecká L, Slouka Z. Swelling induced structural changes of a heterogeneous cation-exchange membrane analyzed by micro-computed tomography. J Memb Sci. 2017;525:195–201.
Veerman J. The effect of the NaCl bulk concentration on the resistance of ion exchange membranes-measuring and modeling. Energies. 2020;13.
Berezina NP, Kononenko NA, Dyomina OA, Gnusin NP. Characterization of ion-exchange membrane materials: properties vs structure. Adv Colloid Interface Sci. 2008;139:3–28.
DOI: https://doi.org/10.15826/chimtech.2021.8.2.05
Copyright (c) 2021 D.V. Davidov, E.N. Nosova, S.A. Loza, A.R. Achoh, A.N. Korzhov, S.S. Melnikov

This work is licensed under a Creative Commons Attribution 4.0 International License.
Chimica Techno Acta, 2014–2025
eISSN 2411-1414
Copyright Notice







