ABO4 type scheelite phases in (Ca/Sr)MoO4 - BiVO4 - Bi2Mo3O12 systems: synthesis, structure and optical properties
Abstract
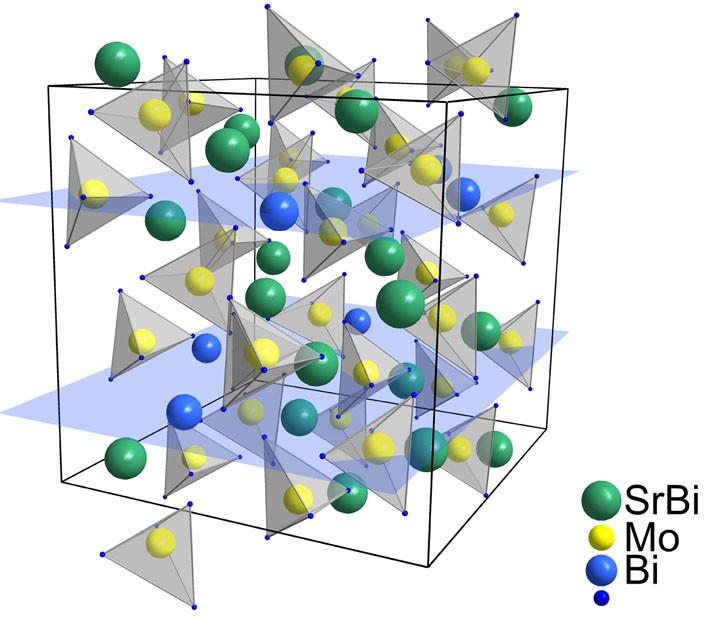
Ca1−1.5x-yBix+yФxMo1-yVyO4 and Sr1−1.5x-yBix+yФxMo1-yVyO4 were synthesized byconvention solid state technique in the range of 550–720 °C. Two wide regions of the solid solutions (ordinary and superstructured scheelite-type phases respectively) were found for each system. The diffuse scatering of homogeneous samples was investigated in the range of 190–1100 nm. Energy gaps calculated with linear approximation of Kubelka-Munk function decreases with bismuth and vanadium content.
Keywords
Full Text:
PDFReferences
Frank M, Smetanin SN, Jelínek M, Vyhlídal D, Kopalkin AA, Shukshin VE, Ivleva LI, Zverev PG, Kubeček V. Synchronously-pumped all-solid-state SrMoO4 Raman laser generating at combined vibrational Raman modes with 26-fold pulse shortening down to 1.4 ps at 1220 nm. Opt Laser Technol. 2019;111:129-33. doi:10.1016/j.optlastec.2018.09.045
Kunzel R, Umisedo NK, Okuno E, Yoshimura EM, Marques AP. Effects of microwave-assisted hydrothermal treatment and beta particles irradiation on the thermoluminescence and optically stimulated luminescence of SrMoO4 powders. Ceram Int. 2020;46(10):15018-26. doi:10.1016/j.ceramint.2020.03.032
Yu H, Shi, X, Huang L, Kang X, Pan D. Solution-deposited and low temperature-annealed Eu3+/Tb3+-doped CaMoO4/SrMoO4 luminescent thin films. J Lumin. 2020;225:117371. doi:10.1016/j.jlumin.2020.117371
Elakkiya V, Sumathi S. Low-temperature synthesis of environment-friendly cool yellow pigment: Ce substituted SrMoO4. Mater Lett. 2019;263:127246. doi:10.1016/j.matlet.2019.127246
Mikhailik VB, Elyashevskyi Yu, Kraus H, Kim HJ, Kapustianyk V, Panasyuk M. Temperature dependence of scintillation properties of SrMoO4. Nucl Instrum Methods Phys Res A. 2015;792:1-5. doi:10.1016/j.nima.2015.04.018
Guo HH, Zhou D, Pang LX, Qi ZM. Microwave dielectric properties of low firing temperature stable scheelite structured (Ca,Bi)(Mo,V)O4 solid solution ceramics for LTCC applications. J Eur Ceram Soc. 2019;39(7):2365-73. doi:10.1016/j.jeurceramsoc.2019.02.010
Yu-Ling Y, Xue-Ming L, Wen-Lin F, Wu-Lin L, Chuan-Yi T. Co-precipitation synthesis and photoluminescence properties of (Ca1−x−yLny)MoO4: xEu3+ (Ln =Y, Gd) red phosphors. J Alloys Compd. 2010;505(1):239-42. doi:10.1016/j.jallcom.2010.06.037
Zhu Y, Zheng G, Dai Z, Zhang L, Ma Y. Photocatalytic and luminescent properties of SrMoO4 phosphors prepared via hydrothermal method with different stirring speeds. J Mater Sci Technol. 2017;33(1):23-39. doi:10.1016/j.jmst.2016.11.019
Yao Z-F, Zheng G-H, Dai Z-X, Zhang L-Y. Synthesis of the Dy: SrMoO4 with high photocatalytic activity under visible light irradiation. Appl Organomet Chem. 2018;32(8):e4412. doi:10.1002/aoc.4412
Wang Y, Xu H, Shao C, Cao J. Doping induced grain size reduction and photocatalytic performance enhancement of SrMoO4:Bi3+. Appl Surf Sci. 2017;392:649-57. doi:10.1016/j.apsusc.2016.09.09
Vidya S, John A, Solomon S, Thomas J. Optical and dielectric properties of SrMoO4 powders prepared by the combustion synthesis method. Adv Mater Res. 2012;1:191-204. doi:10.12989/amr.2012.1.3.191
Cheng J, Liu C, Cao W, Qi M, Shao G. Synthesis and electrical properties of scheelite Ca1-xSmxMoO4+d solid electrolyte ceramics. Mater Res Bull. 2011;46(2):185-9. doi:10.1016/j.materresbull.2010.11.019
Guo J, Randall CA, Zhou D, Zhang G, Zhang C, Jin B, Wang H. Correlation between vibrational modes and dielectric properties in (Ca1−3xBi2xx)MoO4 ceramics. J Eur Ceram Soc. 2015;35(3):4459-64. doi:10.1016/j.jeurceramsoc.2015.08.020
Pang L-X, Sun G-B, Zhou D. Ln2Mo3O12 (Ln = La, Nd): A novel group of low loss microwave dielectric ceramics with low sintering temperature. Mater Lett. 2011;65(2):164-6. doi:10.1016/j.matlet.2010.09.064
Esaka T. Ionic conduction in substituted scheelite-type oxides. Solid State Ionics. 2000;136-137(1-2):1-9. doi:10.1016/s0167-2738(00)00377-5
Yang X, Wang Y, Wang N, Wang S, Gao G. Effects of co-doped Li+ ions on luminescence of CaWO4:Sm3+ nanoparticles. J Mater Sci Mater Electronics. 2014;25:3996-4000. doi:10.1007/s10854-014-2119-4
Jiang P, Gao W, Cong R, Yang T. Structural investigation of the A-site vacancy in scheelites and the luminescence behavior of two continuous solid solutions A1-1.5xEux•0.5xWO4 and A0.64–0.5yEu0.24Liy•0.12–0.5yWO4 (A = Ca, Sr; • = vacancy). Dalton Trans. 2015;44(13):6175-83. doi:10.1039/c5dt00022j
Sleight JAW, Aykan K. New nonstoichiometric molybdate, tungstate, and vanadate catalysts with the scheelite-type structure. J Solid State Chem. 1975;13(4):231-6. doi:10.1016/0022-4596(75)90124-3
Mikhaylovskaya ZA, Abrahams I, Petrova SA, Buyanova ES, Tarakina NV, Piankova DV, Morozova MV. Structural, photocatalytic and electroconductive properties of bismuth-substituted CaMoO4. J Solid State Chem. 2020;291:121627. doi:10.1016/j.jssc.2020.121627
Mikhaylovskaya ZA, Buyanova ES, Petrova SA, Nikitina АА. Sheelite-related strontium molybdates: synthesis and characterization. Chimica Techno Acta 2018;5(4):189-95. doi:10.15826/chimtech.2018.5.4.03
Kay MI, Frazer BC, Almodovar I. Neutron diffraction refinement of CaWO4. J Chem Phys. 1964;40(2):504-506. doi:10.1063/1.1725144
Yao W, Ye J. Photophysical and photocatalytic properties of Ca1-xBixVxMo1-xO4 solid solutions. J Phys Chem B. 2006;110:11188-95. doi:10.1021/jp0608729
Shannon RD. Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Acta Cryst. 1976;A32:751–67. doi:10.1107/S0567739476001551
Verma A, Sharma SK. Rare-earth doped/codoped CaMoO4 phosphors: A candidate for solar spectrum conversion. Solid State Sci. 2019;96:105945. doi:10.1016/j.solidstatesciences.2019.105945
DOI: https://doi.org/10.15826/chimtech.2021.8.2.04
Copyright (c) 2021 Zoya A. Mikhaylovskaya, Elena S. Buyanova, Sofia A. Petrova, Alexandra V. Klimova

This work is licensed under a Creative Commons Attribution 4.0 International License.
Chimica Techno Acta, 2014–2025
eISSN 2411-1414
Copyright Notice







