New possibilities of the functionalization of 6-hydrazino-1,3-dimethyluracils: one-pot synthesis of 5,7-dimethylpyrazolopyrimidine-4,6-dione and 1,3-dimethyl-5-arylidenebarbituric acid derivatives
Abstract
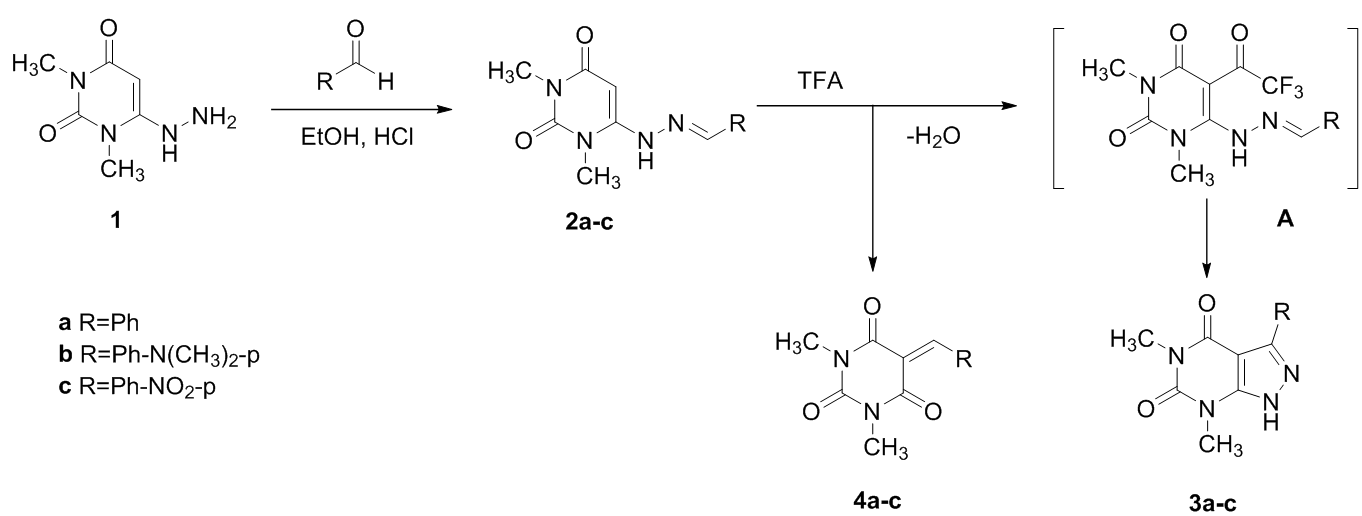
3-aryl-5,7-dimethylpyrazolopyrimidine-4,6-diones and 5-benzylidene-1,3-dimethylpyrimidine-2,4,6-triones were obtained by heating hydrazones of 1,3-dimethyl-6 -hydrazinouraciles in trifluoroacetic acid (TFA). The same compounds were also obtained by heating the hydrazones of 1,3-dimethyl-6-hydrazinouraciles in aqueous ethanol in the presence of hydrochloric acid.
Keywords
Full Text:
PDFReferences
Rashad AE, Shamroukh AH, Ali HS, Abdel-Megeid FME. Some New Pyrazole and Pyrazolopyrimidines: Synthesis and Antimicrobial Evaluation. J Heterocycl Chem. 2013;50:758-65. doi:10.1002/jhet.1550
Eweas AF, Swelam SA, Fathalla OA. Synthesis, anti-microbial evaluation, and molecular modeling of new pyrazolo[3,4-d]pyrimidine derivatives. Med Chem Res. 2012;21:3848-57. doi:10.1007/s00044-011-9911-y
Aggarwal R, Sumran G, Garg N, Aggarwal AA. A regioselective synthesis of some new pyrazol-1′-ylpyrazolo[1,5-a]pyrimidines in aqueous medium and their evaluation as antimicrobial agents. Eur J Med Chem. 2011;46:3038-46. doi:10.1016/j.ejmech.2011.04.041
Rashad AE, Shamroukh AH, Abdel-Megeid RE, Ali SH. Synthesis and Isomerization of Some Novel Pyrazolopyrimidine and Pyrazolotriazolopyrimidine Derivatives. Molecules. 2014;19:5459-69. doi:10.3390/molecules19055459
Gudmundsson KS, Johns BA, Weatherhead AA. Pyrazolopyrimidines and pyrazolotriazines with potent activity against herpesviruses. J Bioorg Med Chem Lett. 2009;19:5689-92. doi:10.1016/j.bmcl.2009.08.009
Yewale SB, Ganorkar SB, Baheti KG, Shelke RU. Novel 3-substituted-1-aryl-5-phenyl-6-anilinopyrazolo[3,4-d]pyrimidin-4-ones: Docking, synthesis and pharmacological evaluation as a potential anti-inflammatory agents. Bioorg Med Chem Lett. 2012;22:6616-20. doi:10.1016/j.bmcl.2012.08.119
El-Tombany AA. Synthesis, anti-inflammatory, and Ulcerogenicity studies of novel substituted and fused pyrazolo[3,4-d]pyrimidin-4-ones. Sci Pharm. 2013;81:393-422. doi:10.3797/scipharm.1211-21
Alcaro S, Artese A, Botta M, Zizzari AT, Orallo F, Ortuso F, Schenone S, Brullo C, Yáñez M. Hit Identification and Biological Evaluation of Anticancer Pyrazolopyrimidines Endowed with Anti-inflammatory Activity. Chem Med Chem. 2010;5:1242-6. doi:10.1002/cmdc.201000165
Hurst DT. An introduction to the chemistry and biochemistry of pyrimidines, purines and pteridines. New York: Wiley&Sons, 1980, 266 p. doi:10.1016/0307-4412(80)90067-9
Ichiba M, Senga K. Reaction of 6-arylidenehydrazino-1,3-dimethyluracils with thionyl chloride leading to purine, thiazolo[4,5-d]pyrimidine, pyrimido[4,5-e][1,3,4]thiadiazine, pyrazolo[3,4-d]pyrimidine, and [1,2,3]thiadiazolo[4,5-d]pyrimidine derivativese. J Het Chem. 1985;22:381-6. doi:10.1002/jhet.5570220233
Kanazawa H, Nishigaki S, Senga K. N-bromosuccinimide in heterocyclic synthesis. Synthesis of pyrazolo[3,4-d]pyrimidines, Pyrimido[5,4-e]-as-triazines, and pyrimido[4,5-c]pyridazines from 6-arylidenehydrazino-1,3-dimethyluracil derivatives. J Het Chem. 1984;21:969-73. doi:10.1002/jhet.5570210408
Yoneda F, Nagamatsu T. The Thermolysis and Photolysis of 6-(Benzylidenehydrazino)uracils. New Syntheses of Pyrazolo[3,4-d]pyrimidines. A Method to Convert Aldehydes to Nitriles. Bull Chem Soc Jap. 1975;48:1484-9. doi:10.1246/bcsj.48.1484
Pfleiderer W, Schuendenhuette KH. Untersuchungen in der Pyrimidinreihe IV. Umsetzungen mit 1,3-dimethyl-4-chlor-uracil. J Lieb Ann Chem. 1958;612:1. doi:10.1002/jlac.19586120117
Pfleaderer W, Blankenhorn G. Eine neue pyrimido-[5,4-e]-as-triazin synthesis. Tetr Lett. 1969;53:4699-702.
Deb ML, Bhuyan PJ. Uncatalysed Knoevenagel condensation in aqueous medium at room temperature. Tetr Let. 2005;46:6453-6. doi:10.1016/j.tetlet.2005.07.111
Shi D, Shi J, Rong S. A Facile and Clean Synthesis of Pyrimidine Derivatives via Three-component Reaction in Aqueous Media. Chin J Chem. 2010;28:791-6. doi:10.1002/cjoc.201090148
DOI: https://doi.org/10.15826/chimtech.2021.8.2.03
Copyright (c) 2021 Yu.A. Azev, O.S. Koptyaeva, T.A. Pospelova

This work is licensed under a Creative Commons Attribution 4.0 International License.
Chimica Techno Acta, 2014–2025
eISSN 2411-1414
Copyright Notice






