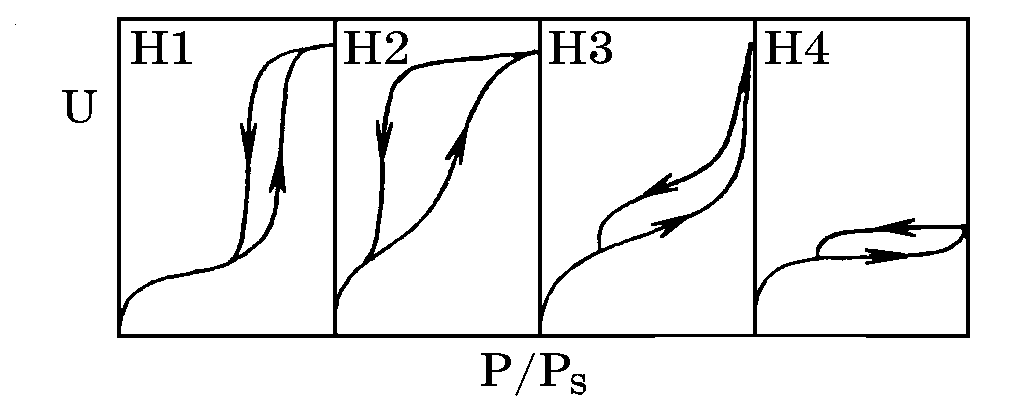
Overview of sorption analysis capabilities for meso- and microporous zeolites nanomaterials
Abstract
Keywords
Full Text:
PDFReferences
Everett D. Manual of Symbols and Terminology for Physicochemical Quantities and Units, Appendix II: Definitions, Terminology and Symbols in Colloid and Surface Chemistry. Pure and Applied Chemistry. 1972;31(4):577-638. doi:10.1351/pac197231040577
Wang G, Wang K, Ren T. Improved analytic methods for coal surface area and pore size distribution determination using 77K nitrogen adsorption experiment. Int J Min Sci Technol. 2014;24(3):329-334. doi:10.1016/j.ijmst.2014.03.007
Gavrilov VY. Fiziko-himicheskie osnovy adsorbcionnogo analiza dispersnyh i poristyh materialov [Physicochemical bases of adsorption analysis of dispersed and porous materials]. Novosibirsk: NCTC; 2007. 67 p. Russian.
Zelenka T. Adsorption and desorption of nitrogen at 77 K on micro- and mesoporous materials: Study of transport kinetics. Journal of Microporous and Mesoporous Materials. 2016;227:202-209. doi:10.1016/j.micromeso.2016.03.009
Nguyen TB, Huang CP, Doong RA, Chen CW, Dong CD. CoO-3D ordered mesoporous carbon nitride (CoO@mpgCN) composite as peroxymonosulfate activator for the degradation of sulfamethoxazole in water. Journal of Hazardous Materials. 2021;401:123320. doi:10.1016/j.jhazmat.2020.123326
Dong GY, Tian GY ,Gong LL, Tang QG. Mesoporous zinc silicate composites derived from iron ore tailings for highly efficient dye removal: Structure and morphology evolution. Journal of Microporous and Mesoporous Materials. 2020;305:110352. doi:10.1016/j.micromeso.2020.110352
Hadis M, Jafar T, Babak M. Enhanced nitrogen adsorption capacity on Ca2+ ion-exchanged hierarchical X zeolite. Separation and Purification Technology. 2021;264:118442. doi:10.1016/j.seppur.2021.118442
Kononova IE, Maraeva EV, Skornikova SA, Moshnikov VA. Influence of binder on porous structure of zeolite compositions and their catalytic activity. Glass Physics and Chemistry. 2020;46(2):162-169. doi:10.1134/S1087659620020066
Gracheva IE, Moshnikov VA, Maraeva EV, Karpova SS, Alexsandrova OA, Alekseyev NI, Kuznetsov VV, Olchowikc G, Semenov KN, Startseva AV, Sitnikov AV, Olchowikc JM. Nanostructured materials obtained under conditions of hierarchical self-assembly and modified by derivative forms of fullerenes. Journal of non-crystalline solids. 2012;358(2):433-439. doi:10.1016/j.jnoncrysol.2011.10.020
Maraeva EV, Permiakov NV, Kedruk YY, Gritsenko LV, Abdullin KhA. Creating a virtual device for processing the results of sorption measurements in the study of zinc oxide nanorods. 2020;7(4):154-158. doi:10.15826/chimtech.2020.7.4.03
DOI: https://doi.org/10.15826/chimtech.2021.8.3.02
Copyright (c) 2021 Saida Tokmeilova, Evgeniya Vladimirovna Maraeva

This work is licensed under a Creative Commons Attribution 4.0 International License.
Chimica Techno Acta, 2014–2025
eISSN 2411-1414
Copyright Notice







