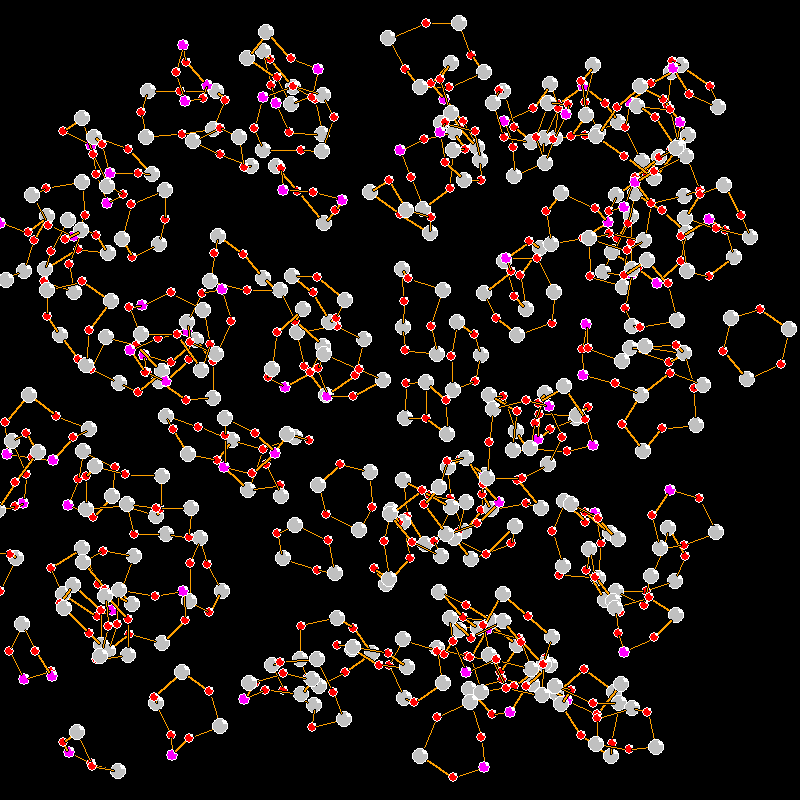
Structural changes in V2O5-P2O5 glasses: non-constant force field molecular dynamics and IR spectroscopy
Abstract
Keywords
Full Text:
PDFReferences
El-Damrawi G, Abdelghany AM, Hassan AK, Faroun B. Conductivity and morphological studies on iron borosilicate glasses. J Non Cryst Solids. 2020;545:120233. doi:10.1016/j.jnoncrysol.2020.120233
Kaur N, Khanna A, Fábián M, Dutt S. Structural and electrical characterization of semiconducting xCuO-(100-x)TeO2 glasses. J Non Cryst Solids. 2020;534:119884. doi:10.1016/j.jnoncrysol.2020.119884
Ningthemcha RKN, Biswas D, Singh YB, Sarkar D, Mondal R, Mandal D, et al. Temperature and frequency dependent electrical conductivity and dielectric relaxation of mixed transition metal doped bismuth-phosphate semiconducting glassy systems. Mater Chem Phys. 2020;249:123207. doi:10.1016/j.matchemphys.2020.123207
Afyon S, Krumeich F, Mensing C, Borgschulte A, Nesper R. New high capacity cathode materials for rechargeable Li-ion batteries: Vanadate-borate glasses. Sci Rep [Internet]. 2014;4:7113. doi:10.1038/srep07113
Kaur N, Khanna A, Kaur R, Ruchika, Salhotra D. Synthesis and characterization of vanadium and iron tellurite glasses for applications as thermal sensors. Solid State Sci. 2021;114:106564. doi:10.1016/j.solidstatesciences.2021.106564
Li X, Xiao Z, Luo M, Dong X, Du T, Wang Y. Low melting glasses in ZnO-Fe2O3-P2O5 system with high chemical durability and thermal stability for sealing or waste immobilization. J Non Cryst Solids. 2017;469:62–9. doi:10.1016/j.jnoncrysol.2017.04.023
Raskovalov AA, Saetova NS. All-solid-state batteries based on glass-ceramic lithium vanadate. Solid Electrolytes Adv Appl. Springer; 2019. p. 297–334.
Saiko IA, Saetova NS, Raskovalov AA, Il’ina EA, Molchanova NG, Kadyrova NI. Hopping conductivity in V2O5-P2O5 glasses: Experiment and non-constant force field molecular dynamics. Solid State Ionics. 2020;345:115180. doi:10.1016/j.ssi.2019.115180
Hoppe U, Wyckoff NP, Schmitt ML, Brow RK, Schöps A, Hannon AC. Structure of V2O5-P2O5 glasses by X-ray and neutron diffraction. J Non Cryst Solids. 2012;358:328–36. doi:10.1016/j.jnoncrysol.2011.09.038
Aoyagi T, Kohara S, Naito T, Onodera Y, Kodama M, Onodera T, et al. Controlling oxygen coordination and valence of network forming cations. Sci Rep. 2020;10:7178. doi:10.1038/s41598-020-63786-y
Nabavi M, Sanchez C, Livage J. Structure and properties of amorphous V2O5. Philos Mag B Phys Condens Matter; Stat Mech Electron Opt Magn Prop. 1991;63:941–53. doi:10.1080/13642819108205549
Feltz A, Unger B. Redox reactions in condensed oxide systems II. Variation of the structure of vanadium phosphate glasses in dependence on the oxidation state of vanadium. J Non Cryst Solids. 1985;72:335–43. doi:10.1016/0022-3093(85)90188-7
Wadsworth M, France PW. Nmr quadrupole interactions in vanadium phosphate glass. J Phys C Solid State Phys. 1986;19:7129–43. doi:10.1088/0022-3719/19/36/005
Landsberger FR, Bray PJ. Magnetic resonance study of the V2O5-P2O5 semiconducting glass system. J Chem Phys. 1970;53:2757–68. doi:10.1063/1.1674400
Livage J, Pineau P, Leroy MC, Michaud M. Semiconducting vanadium phosphate glasses. Phys status solidi. 1977;39:73–8. doi:10.1002/pssa.2210390107
Bhargava RN, Condrate RA. Vibrational spectra of VPO5 crystal phases and related glasses. Appl Spectrosc. 1977;31:230–60. doi:10.1366/000370277774463742
Gopal R, Calvo C. Crystal structure of βVPO5. J Solid State Chem. 1972;5:432–5. doi:10.1016/0022-4596(72)90089-8
Sakurai Y, Yamaki J. Correlation between microstructure and electrochemical behavior of amorphous V2O5-P2O5 in lithium cells. ChemInform. 1988;19:791–6. doi:10.1002/chin.198831013
Saetova NS, Raskovalov AA, Il’ina EA, Antonov BD, Grzhego-rzhevskii KV. Structure and electrical conductivity of glasses 30Na2O–xV2O5–(70–x)B2O3: Experiment and molecular dynamics with self-assembly elements. Russ J Inorg Chem. 2021;66:313–23. doi:10.1134/S003602362103013X
Wojdyr M. Fityk: A general-purpose peak fitting program. J Appl Crystallogr. 2010;43:1126–8. doi:10.1107/S0021889810030499
Raskovalov AA. azTotMD: Software for non-constant force field molecular dynamics. SoftwareX. 2019;10:100233. doi:10.1016/j.softx.2019.04.005
Pedone A, Malavasi G, Menziani MC, Cormack AN, Segre U. A new self-consistent empirical interatomic potential model for oxides, silicates, and silicas-based glasses. J Phys Chem B. 2006;110:11780–95. doi:10.1021/jp0611018
Fennell CJ, Gezelter JD. Is the Ewald summation still necessary? Pairwise alternatives to the accepted standard for long-range electrostatics. J Chem Phys. 2006;124:234104. doi:10.1063/1.2206581
Stefan R, Simedru D, Popa A, Ardelean I. Structural investigations of V2O5-P2O5-CaO glass system by FT-IR and EPR spectroscopies. J Mater Sci. 2012;47:3746–51. doi:10.1007/s10853-011-6225-x
Vedeanu N, Stanescu R, Filip S, Ardelean I, Cozar O. IR and ESR investigations on V2O5-P2O5-BaO glass system with opto-electronic potential. J Non Cryst Solids. 2012;358:1881–5. doi:10.1016/j.jnoncrysol.2012.05.010
Ahsan MR, Uddin MA, Mortuza MG. Infrared study of the effect of P2O5 in the structure of lead silicate glasses. Indian J Pure Appl Phys. CSIR; 2005;43:89–99.
Sindhu S, Sanghi S, Agarwal A, Seth VP, Kishore N. Structural, optical, physical and electrical properties of V2O5·SrO·B2O3 glasses. Spectrochim Acta - Part A Mol Biomol Spectrosc. 2006;64:196–204. doi:10.1016/j.saa.2005.06.039
Sindhu S, Sanghi S, Agarwal A, Sonam, Seth VP, Kishore N. The role of V2O5 in the modification of structural, optical and electrical properties of vanadium barium borate glasses. Phys B Condens Matter. 2005;365:65–75. doi:10.1016/j.physb.2005.04.037
Yadav AK, Singh P. A review of the structures of oxide glasses by Raman spectroscopy. RSC Adv. 2015;5:67583–609. doi:10.1039/c5ra13043c
Lewandowska R, Krasowski K, Bacewicz R, Garbarczyk JE. Studies of silver-vanadate superionic glasses using Raman spectroscopy. Solid State Ionics. 1999;119:229–34. doi:10.1016/S0167-2738(98)00508-6
Vedeanu NS, Cozar IB, Stanescu R, Stefan R, Vodnar D, Cozar O. Structural investigation of V2O5-P2O5-K2O glass system with antibacterial potential. Bull Mater Sci. 2016;39:697–702. doi:10.1007/s12034-016-1214-y
Garbarczyk JE, MacHowski P, Wasiucionek M, Tykarski L, Bacewicz R, Aleksiejuk A. Studies of silver-vanadate-phosphate glasses by Raman, EPR and impedance spectroscopy methods. Solid State Ionics. 2000;136–137:1077–83. doi:10.1016/S0167-2738(00)00504-X
Khattak GD, Mekki A, Siddiqui MN. Compositional depend-ence of DC electrical conductivity of SrO-vanadate glasses. Solid State Ionics. 2012;211:5–11. doi:10.1016/j.ssi.2012.01.012
Toloman D, Biris AR, Maniu D, Bratu I, Giurgiu LM, Biris AS, et al. Phosphate glassy network depolymerization induced by CaO doping. Part Sci Technol. 2010;28:226–35. doi:10.1080/02726351.2010.481581
Galliano PG, López JMP, Varetti EL, Sobrados I, Sanz J. Analysis by nuclear magnetic resonance and raman spectroscopies of the structure of bioactive alkaline-earth silicophosphate glasses. Mater Res Bull. 1994;29:1297–306. doi:10.1016/0025-5408(94)90154-6
Tatsumisago M, Kowada Y, Minami T. Raman spectra of rapidly quenched glasses and melts containing large amounts of Li2O. J Non Cryst Solids. 1992;150:207–11. doi:10.1016/0022-3093(92)90124-3
Abd El-Moneim A. DTA and IR absorption spectra of vanadi-um tellurite glasses. Mater Chem Phys. 2002;73:318–22. doi:10.1016/S0254-0584(01)00355-8
Majjane A, Chahine A, Et-Tabirou M, Echchahed B, Do TO, Breen PM. X-ray photoelectron spectroscopy (XPS) and FTIR studies of vanadium barium phosphate glasses. Mater Chem Phys. 2014;143:779–87. doi:10.1016/j.matchemphys.2013.10.013
Karabulut M, Metwalli E, Brow RK. Structure and properties of lanthanum-aluminum-phosphate glasses. J Non Cryst Solids. 2001;283:211–9. doi:10.1016/S0022-3093(01)00420-3
Pires RA, Abrahams I, Nunes TG, Hawkes GE. The role of alumina in aluminoborosilicate glasses for use in glass-ionomer cements. J Mater Chem. 2009;19:3652–60. doi:10.1039/b822285a
Metwalli EE, Brow RK, Stover FS. Cation effects on anion distributions in aluminophosphate glasses. J Am Ceram Soc. 2001;84:1025–32. doi:10.1111/j.1151-2916.2001.tb00785.x
Abou Neel EA, Chrzanowski W, Pickup DM, O’Dell LA, Mordan NJ, Newport RJ, et al. Structure and properties of strontium-doped phosphate-based glasses. J R Soc Interface. 2009;6:435–46. doi:10.1098/rsif.2008.0348
Rao KJ, Sobha KC, Kumar S. Infrared and Raman spectroscopic studies of glasses with NASICON-type chemistry. Proc Indian Acad Sci Chem Sci. 2001;497–514. doi:10.1007/BF02708786
Dayanand C, Bhikshamaiah G, Java Tyagaraju V, Salagram M, Krishna Murthy ASR. Structural investigations of phosphate glasses: A detailed infrared study of the x(PbO)-(1-x)P2O5 vitreous system. J. Mater. Sci. 1996;1945–67. doi:10.1007/BF00356615
Tsai PP, Greenblatt M. Lithium ion conducting glasses in the system LiCl-Li2O-P2O5-SiO2. J Non Cryst Solids. 1988;103:101–7. doi:10.1016/0022-3093(88)90421-8
Saad M, Stambouli W, Sdiri N, Elhouichet H. Effect of mixed sodium and vanadium on the electric and dielectric proper-ties of zinc phosphate glass. Mater Res Bull. 2017;89:224–31. doi:10.1016/j.materresbull.2017.01.043
Magdas DA, Vedeanu NS, Toloman D. Study on the effect of vanadium oxide in calcium phosphate glasses by Raman, IR and UV-vis spectroscopy. J Non Cryst Solids. 2015;428:151–5. doi:10.1016/j.jnoncrysol.2015.08.012
Marzouk MA, Elbatal FH, Abdelghany AM. Ultraviolet and infrared absorption spectra of Cr2O3 doped - sodium meta-phosphate, lead metaphosphate and zinc metaphosphate glasses and effects of gamma irradiation: A comparative study. Spectrochim Acta - Part A Mol Biomol Spectrosc. 2013;114:658–67. doi:10.1016/j.saa.2013.05.093
Zachariasen WH. The atomic arrangement in glass. J Am Chem Soc. 1932;54:3841–51. doi:10.1021/ja01349a006
DOI: https://doi.org/10.15826/chimtech.2021.8.2.11
Copyright (c) 2020 A.A. Raskovalov, N.S. Saetova, I.S. Popov

This work is licensed under a Creative Commons Attribution 4.0 International License.
Chimica Techno Acta, 2014–2025
eISSN 2411-1414
Copyright Notice







