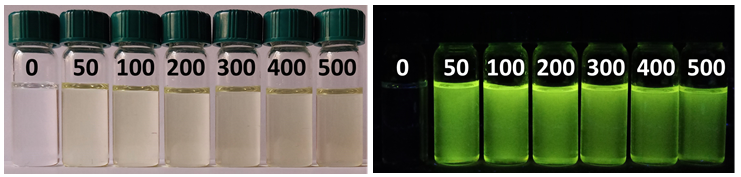
Sensitivity of the phenoxy derivatives of 2,4-dihydro-5H-[1,2,3]triazolo[4,5-d]pyrimidin-5-ones to acidic and basic stimuli
Abstract
Keywords
Full Text:
PDFReferences
Qian J, Zhang Y, Liu X, Xia J. Carbazole and fluorene polyaniline derivatives: Synthesis, properties and application as multiple stimuli-responsive fluorescent chemosensor. Talanta. 2019;204:592–601. doi:10.1016/j.talanta.2019.06.038
Valeur B, Berberan-Santos MN. Molecular Fluorescence. Weinheim: Wiley VCH; 2013. 592 p.
Srinivasan R, Phillips TE, Bargeron CB, Carlson MA, Schemm ER, Saffarian HM. Embedded micro-sensor for monitoring pH in concrete structures. Proc SPIE. 2000;3988:40–4. doi:10.1117/12.383169
Basheer PAM, Grattan KTV, Sun T, Long AE, McPolin D, Xie W. Fibre optic chemical sensor systems for monitoring pH changes in concrete. Proc SPIE. 2004;5586:144–53. doi:10.1117/12.601198
Hecht M, Kraus W, Rurack K. A highly fluorescent pH sensing membrane for the alkaline pH range incorporating a BODIPY dye. Analyst. 2013;138(1):325–32. doi:10.1039/C2AN35860C
Gotor R, Ashokkumar P, Hech M, Keil K, Rurack K. Optical pH sensor covering the range from pH 0-14 compatible with mobile-device readout and based on a set of rationally designed indicator dyes. Anal Chem. 2017;89(16):8437–44. doi:10.1021/acs.analchem.7b01903
Pfeifer D, Klimart I, Borisov SM. Ultra-bright red-emitting photostable perylene bisimide dyes: new indicators for ratiometric sensing of high pH or carbon dioxide. Chem Eur J. 2018;24:10711–20. doi:10.1002/chem.201800867
Eltyshev AK, Suntsova PO, Karmatskaia KD, Taniya OS, Slepukhin PA, Benassi E, Belskaya NP. An effective and facile synthesis of new blue fluorophores on the basis of an 8-azapurine core. Org Biomol Chem. 2018;16(48):9420–29. doi:10.1039/C8OB02644K
Li X, Peng Y, Liu H , Xu Y , Wang X , Zhang C, Ma X. Comparative studies on the interaction of nine flavonoids with trypsin. Spectrochim Acta A. 2020;238:118440. doi:10.1016/j.saa.2020.118440
Yao J, He Y, Su N, Bharath SR, Tao Y, Jin JM, Chen W, Song H, Tang SY. Developing a highly efficient hydroxytyrosol whole-cell catalyst by de-bottlenecking rate-limiting steps. Nat Commun. 2020;11:1515. doi:10.1038/s41467-020-14918-5
Jadhav AS, Carreira-Blanco C, Fernández B, González Fernández S, Malkhede DD, Mosquera MM, Ríos Rodríguez MC, Rodríguez-Prieto F. Firefly luciferin precursor 2-cyano-6-hydroxybenzothiazole: Fluorescence à la carte controlled by solvent and acidity. Dyes Pigm. 2020;177:108285. doi:10.1016/j.dyepig.2020.108285
Aysha TS, El-Sedik MS, Mohamed MBI, Gaballah ST, Kamel MM. Dyes Pigm 2019;170:107549. doi:10.1016/j.dyepig.2019.107549
Zhang J, Lia J, Chen B, Kan J, Jiang T, Zhang W, Yue J, Zhou J. An off-on fluorescent probe for real-time sensing the fluctuations of intracellular pH values in biological processes. Dyes Pigm. 2019;170:107620. doi:10.1016/j.dyepig.2019.107620
Bondar K, Bokan M, Gellerman G, Patsenker LD. Water-soluble 4-hydroxystyryl and 4-hydroxyphenyl-butadienyls dyes with switchable fluorescence. Dyes Pigm. 2020;172:107801. doi:10.1016/j.dyepig.2019.107801
Seo MH, Han J, Jin Z, Lee DW, Park HS, Kim HS. Controlled and Oriented Immobilization of Protein by Site-Specific Incorporation of Unnatural Amino Acid. Anal Chem. 2011;83(8):2841–45. doi:10.1021/ac103334b
Eichelbaum K, Winter M, Berriel Diaz M, Herzig S, Krijgsveld J. Selective enrichment of newly synthesized proteins for quantitative secretome analysis. Nat Biotechnol. 2012;30:984–90. doi:10.1038/nbt.2356
Raliski BK, Howard CA, Young DD. Site-Specific Protein Immobilization Using Unnatural Amino Acids. Bioconjugate Chem. 2014;25:1916-20. doi:10.1021/bc500443h
Hutchins BM, Kazane SA, Staflin K, Forsyth JS, Felding-Habermann B, Schultz PG, Smider VV. Site-Specific Coupling and Sterically Controlled Formation of Multimeric Antibody Fab Fragments with Unnatural Amino Acids. J Mol Biol. 2011;406:595–603. doi:10.1016/j.jmb.2011.01.011
Young DD, Jockush S, Turro NJ, Schultz PG. Synthetase polyspecificity as a tool to modulate protein function. Bioorg. Med Chem Lett. 2011;21:7502–04. doi:10.1016/j.bmcl.2011.09.108
Gorduk S. Highly soluble HOPEMP-functionalized phthalocyanines for photodynamic activity: Photophysical, photochemical and aggregation properties. J Mol Struct. 2020;1217:128478. doi:10.1016/j.molstruc.2020.128478
Eltyshev AK, Minin AS, Smoliuk LT, Benassi E, Belskaya NP. 2-Aryl-2,4-dihydro-5H-[1,2,3]triazolo[4,5-d]pyrimidin-5-ones as a New Platform for the Design and Synthesis of Biosensors and Chemosensors. Eur J Org Chem. 2020;2020:316–29. doi:10.1002/ejoc.201901582
DOI: https://doi.org/10.15826/chimtech.2021.8.3.03
Copyright (c) 2021 Alexander K. Eltyshev, Nataliya P. Belskaya

This work is licensed under a Creative Commons Attribution 4.0 International License.
Chimica Techno Acta, 2014–2025
eISSN 2411-1414
Copyright Notice







