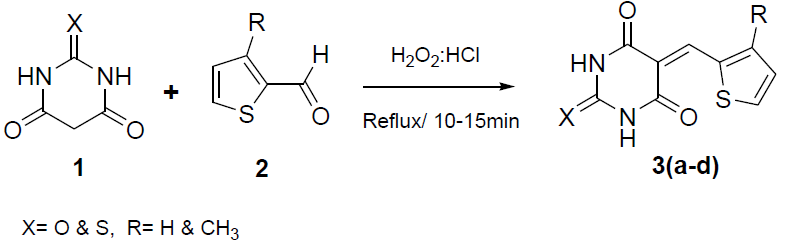
Facile synthesis of some 5-(3-substituted-thiophene)-pyrimidine derivatives and their pharmacological and computational studies
Abstract
Keywords
Full Text:
PDFReferences
Singh PK, Silakari O. The current status of O-heterocycles: A synthetic and medicinal overview. Med Chem. 2018;13:1071–1087. doi:10.1002/cmdc.201800119
Pathania S, Narang RK, Rawal RK. Role of sulphur-heterocycles in medicinal chemistry: An update. Eur J Med Chem. 2019;180:486–508. doi:10.1016/j.ejmech.2019.07.043
Venkatesh T, Bodke YD, Manjunatha B, Kumar SR. Synthesis, antitubercular activity and molecular docking study of substituted [1,3]dioxino[4,5-d] pyrimidine derivatives via facile CAN catalyzed Biginelli reaction. Nucleosides, Nucleotides and Nucleic Acids. 2021:1–13. doi:10.1080/15257770.2021.1972310
Sravanthi B, Kaviarasan L, Praveen TK, Sai Kiran PSS, Pavankumar C, Gowramma B. Synthesis and pharmacological evaluation of 1,3,4-thiadiazole bearing pyrimidine derivatives as STAT3 inhibitor for treatment of breast cancer. J Iran Chem Soc. 2020;17:2359–2370. doi:10.1007/s13738-020-01932-z
Kang D, Feng D, Ginex T, Zou J, Wei F, Zhao T, Huang B, Sun Y, Desta S, Clercq ED, Pannecouque C, Zhan P, Liu X. Exploring the hydrophobic channel of NNIBP leads to the discovery of novel piperidinesubstituted thiophene[3,2-d]pyrimidine derivatives as potent HIV-1 NNRTIs. Acta Pharm Sin B. 2020;10:878–894. doi:10.1016/j.apsb.2019.08.013
Prasad P, Anirudhdha GK and Patel MP. Microwave assisted one-pot synthetic route to imidazo[1,2-a]pyrimidine derivatives of imidazo/triazole clubbed pyrazole and their pharmacological screening. New J Chem. 2018;42:12666–12676. doi:10.1039/C8NJ00670A
Zia UHK, Arif UK, Pingyu W, Yongmei C, Dandan K, Shafiullah K, Kamran T. In vitro pharmacological screening of three newly synthesised pyrimidine derivatives. Nat Prod Res. 2015;29:933–938. doi:10.1080/14786419.2014.964707
Ghada SM. New Potential Antitumor Pyrimidine Derivatives: Synthesis and Cytotoxicity Evaluation. Polycycl Aromat Compd. 2021:1–17. doi:10.1080/10406638.2021.1936086
Zhen X, Cilong C, Lingjia Z, Zhihui Z, Qian Z, Feiyi Y, Zunhua Y, Pengwu Z, Shan X, Wufu Z. Discovery of thiapyran-pyrimidine derivatives as potential EGFR inhibitors. Bioorg Med Chem. 2020;28:1–13. doi:10.1016/j.bmc.2020.115669
Zhen X, Zhihui Z, Cilong C, Qian Z, Lingjia Z, Zunhua Y, Xin Li, Liying Yu, Pengwu Z, Shan Xu, Wufu Z. Design, synthesis and antitumor activity of novel thiophene-pyrimidine derivatives as EGFR inhibitors overcoming T790M and L858R/T790M mutations. Eur J Med Chem. 2020;203:1–43. doi:10.1016/j.ejmech.2020.112511
Elshaymaa IE. Thieno[2,3-d]pyrimidine derivatives: Synthetic approaches and their FLT3 kinase inhibition. J Heterocyclic Chem. 2020;57:2067–2078. doi:10.1002/jhet.3934
Khalil NA, Ahmed EM, Zaher AF, El-Zoghbi MS, Sobh EA. Synthesis of certain benzothieno[3,2-d]pyrimidine derivatives as a selective SIRT2 inhibitors. Eur J Med Chem. 2020;187:1–56. doi:10.1016/j.ejmech.2019.111926
Shipilovskikh SA, Rubtsov AE. One-Pot Synthesis of Thieno[3,2-e]pyrrolo[1,2-a]pyrimidine Derivative Scaffold: A Valuable Source of PARP-1 Inhibitors. J Org Chem. 2019;84:15788–15796. doi:10.1021/acs.joc.9b00711
Lei S, Chengjuan C, Xueting H, Hao H, Manman W, Jianqiu Z, Yile Y, Jianming L, Tiantai Z, Dayong Z. Design, synthesis, and pharmacological evaluation of 4- or 6-phenylpyrimidine derivatives as novel and selective Janus kinase 3 inhibitors. Eur J Med Chem. 2020;191:1–18. doi:10.1016/j.ejmech.2020.112148
Giuseppe R, Loredana S, Valeria P, Marialuisa C, Sebastiano I, Emanuele A, Mario S, Alfredo C, Antonio R, Giuseppe F, Maria NM. [1]Benzothieno[3,2-d]pyrimidine derivatives as ligands for the serotonergic 5-HT7 receptor. Eur J Med Chem. 2019;183:1–16. doi:10.1016/j.ejmech.2019.111690
Ali I, Wani WA, Saleem K, Hsieh MF. Anticancer metallodrugs of glutamic acid sulphonamides: in silico, DNA binding, hemolysis and anticancer studies. RSC Adv. 2014;4:29629–29641. doi:10.1039/C4RA02570A
Aarti A, Archana K, Dr. Amarjit KA, Dr. Nithish C, Yudhvir S. Recent advances made on anticancer drugs the therapeutic potential of the aromatic heterocyclic compounds. Int J Pharm Sci Rev Res. 2019;58:104–113.
Jawaid A, Ahsan AK, Zulphikar Ali, Rafi H, Shahar YM. Structure-activity relationship (SAR) study and design strategies of nitrogen-containing heterocyclic moieties for their anti-cancer activities. Eur J Med Chem. 2017;125:143–189. doi:10.1016/j.ejmech.2016.09.023
Prabodh CS, Kushal KB, Archana S, Diksha S, Aakash D. Thiazole-containing compounds as therapeutic targets for cancer therapy. Eur J Med Chem. 2020;188:1–47. doi:10.1016/j.ejmech.2019.112016
Garima M, Sumitra N, Pramod KS. Cancer: An overview. J Cancer Res. 2015;8:1–9. doi:10.5829/idosi.ajcr.2015.8.1.9336
Nathan MR and Schmid P. A review of fulvestrant in breast cancer. Oncol Ther. 2017;5:17–29. doi:10.1007/s40487-017-0046-2
Akhtar J, Khan AA, Ali Z, Haider R, Yar MS. Structure-activity relationship (SAR) study and design strategies of nitrogen containing heterocyclic moieties for their anticancer activities. Eur J Med Chem. 2016;125:143–189. doi:10.1016/ j.ejmech.2016.09.023
Koohshari M, Dabiri M, Salehi P. Catalyst-free domino reaction in water/ethanol: an efficient, regio- and chemoselective one-pot multi-component synthesis of pyranopyrazole derivatives. RSC Adv. 2014;4:10669–10671. doi:10.1039/C3RA47639A
Daniel RB, De Visser SP, Shaik S, Neumann R. Electrophilic aromatic chlorination and haloperoxidation of chloride catalyzed by polyfluorinated alcohols: a new manifestation of template catalysis. J Am Chem Soc. 2003;125:12116–12117. doi:10.1021/ja0364524
Venkatesh T, Bodke YD, Nagaraj K, Kumar SR. One-pot synthesis of novel substituted phenyl-1,5-dihydro-2h-benzo[4,5]thiazolo[3,2-a]pyrimido[4,5-d]pyrimidine derivatives as potent antimicrobial agents. Med Chem. 2018;8:1–7. doi:10.4172/2161-0444.1000488
Venkatesh T, Bodke YD, Kenchappa R, Telkar S. Synthesis, antimicrobial and antioxidant activity of chalcone derivatives containing thiobarbitone nucleus. Med Chem. 2016;6:440–448. doi:10.4172/2161-0444.1000383
Sukanya SH, Venkatesh T, Kumar SR, Bodke YD. Facile TiO2 NPs catalysed synthesis of substituted-4-Hydroxy/methoxy benzylidene derivatives as potent antioxidant and anti-tubercular agents. Chem Data Collect. 2021;33:1–16. doi:10.1016/j.cdc.2021.100713
Venkatesh T, Bodke YD, Joy NM, Vinoda BM, Shiralgi Y, Dhananjaya BL. Synthesis of some novel 5,7-disubstituted-2-phenyl-5H-[1,3,4]thiadiazolo[3,2-a]pyrimidine derivatives and evaluation of their biological activity. Lett Org Chem. 2016;13:661–671. doi:10.2174/1570178613666161017123123
Sukanya SH, Venkatesh T, Aditya Rao SJ, Joy MN. Efficient L-Proline catalyzed synthesis of some new (4-substituted-phenyl)-1,5-dihydro-2H-pyrimido[4,5-d][1,3]thiazolo[3,2a]-pyrimidine-2,4(3H)-diones bearing thiazolopyrimidine derivatives and evaluation of their pharmacological activities. J Mol Struct. 2021;1247:1–13. doi:10.1016/j.molstruc.2021.131324
Shivakumara N, Murali Krishna P. Synthesis, Spectral Characterization, and Evaluation of their DNA interactions. Curr Chem Lett. 2019;8:157–168. doi:10.5267/j.ccl.2019.004.004
Ali I, Mukhtar SD, Hsieh MF, Alothman ZA, Alwarthan A. Facile synthesis of indole heterocyclic compounds based micellarnano anticancer drugs. RSC Adv. 2018;8:37905–37914. doi:10.1039/c8ra07060a
Wolfe A, Shimer GH, Meehan T. Polycyclic aromatic hydrocarbons physically intercalate into duplex regions of denatured DNA. Biochemistry. 1987;26:6392–6396.
Lipinski CA, Lombardo F, Dominy BW, Feeney PJ. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv Drug Deliv Rev. 1997;23:3–25. doi:10.1016/S0169-409X(96)00423-1
Aditya R, Venugopal T, Jayanna N, Paramesha M, Ramesh C. Bioactive isolates of Morus species as antibacterial agents and their insilico profiling. Lett. Drug Des Discov. 2020;17:1–9. doi:10.2174/1570180817999201104120815
Jarrahpour A, Motamedifar M, Zarei M, Youssoufi MH, Mimouni M, Chohan ZH. Petra, Osiris, and Molinspiration together as a guide in drug design: Predictions and correlation structure/antibacterial activity relationships of new n-sulfonyl monocyclic β-Lactams. Phosphorus Sulfur Silicon Relat Elem. 2010;185:491–497. doi:10.1080/10426500902953953
Raghavendra S, Aditya Rao SJ, Kumar V, Ramesh CK. Multiple ligand simultaneous docking (MLSD): A novel approach to study the effect of inhibitors on substrate binding to PPO. Comput Biol Chem. 2015;59:81–86. doi:10.1016/j.compbiolchem.2015.09.008
Sander T, Freyss J, Von Korff M, Rufener C. DataWarrior: An open-source program for chemistry aware data visualization and analysis. J Chem Inf Model 2015;55:460–473. doi:10.1021/ci500588j
Venkatesh T, Bodke YD, Aditya Rao SJ. Facile CAN catalyzed one pot synthesis of novel indol-5,8-pyrimido[4,5-d]pyrimidine derivatives and their pharmacological study. Chem Data Collect. 2020;25:1–13. doi:10.1016/j.cdc.2019.100335
Cheng F, Li W, Zhou Y, Shen J, Wu Z, Liu G. AdmetSAR. A comprehensive source and free tool for assessment of chemical ADMET properties. J Chem Inf Model. 2012;52:3099–3105. doi:10.1021/ci300367a
Trott O, Olson JA. AutoDockVina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization and multithreading. J Comput Chem. 2010;31:455–461. doi:10.1002/jcc.21334
Aditya Rao SJ, Ramesh CK, Raghavendra S, Paramesha M. Dehydroabietylamine, A diterpene from carthamus tinctorious L. showing antibacterial and anthelmintic effects with computational evidence. Curr Comput Aided Drug Des. 2020;16:231–237. doi:10.2174/1573409915666190301142811
Aditya Rao SJ, Shivayogi SM, Satyanarayana JK, Kumaran RC. Characterization of isolated compounds from Morus spp. and their biological activity as anticancer molecules. Bioimpacts 2021;11:1–11. doi:10.34172/bi.2021.09
Frisch MJEA, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Nakatsuji H. Gaussian 09, Revision d. 01, Gaussian. Inc. Wallingford CT 2009;201.
Becke AD. A new mixing of Hartree-Fock and local density-functional theories. J Chem Phys. 1993;98:1372–1377.
Hanwell MD, Curtis DE, Lonie DC, Vandermeersch T, Zurek E, Hutchison GR. Avogadro an advanced semantic chemical editor, visualization, and analysis platform. Aust J Chem. 2012;4:1–33. doi:10.1186/1758-2946-4-17
Dighore NR, Anandgaonker PL, Gaikwad ST, Rajbhoj AS. Solvent free green synthesis of 5-arylidine barbituric acid derivatives catalyzed by copper oxide nanoparticles. Res J Chem Sci. 2014;4:93–98.
Khurana JM, Kanika Vij. Nickel nanoparticles catalyzed knoevenagel condensation of aromatic aldehydes with barbituric acids and 2-thiobarbituric acids. Catal Lett. 2010;138:104–110. doi:10.1007/s10562-010-0376-2
Fattahi M, Davoodnia A, Pordel M. Efficient one-pot synthesis of some new pyrimido[5,4:5,6]pyrido[2,3-d]pyrimidines catalyzed by magnetically recyclable Fe3O4 nanoparticles. Russ J Gen Chem. 2017;87:863–867. doi:10.1134/S1070363217040326
Thirupathi G, Venkatanarayana M, Dubey PK, BharathiKumari Y. Facile and green syntheses of 5-arylidene-pyrimidine-2,4,6-triones and 5-arylidene-2-thioxo-dihydro-pyrimidine-4, 6-diones using L-tyrosine as an efficient and eco-friendly catalyst in aqueous medium. Chem Sci Trans. 2013;2:441–446. doi:10.7598/cst2013.385
Tai Li J, Guang Dai H, Liu D, Shuang Li T. Efficient method for synthesis of the derivatives of 5-arylidene barbituric acid catalyzed by aminosulfonic acid with grinding. Synth Commun. 2006;36:789–794. doi:10.1080/00397910500451324
Hu Y, Chen ZC, Le ZG, Zheng QG. Organic reactions in ionic liquids: ionic liquid promoted knoevenagel condensation of aromatic aldehydes with (2-thio) barbituric acid. Synth Commun. 2004;34:4521–4529. doi:10.1081/SCC-200043210
Shabeer M, Barbosa LCA, Karak M, Coelho ACS. Thiobarbiturates as potential antifungal agents to control human infections caused by Candida and Cryptococcus species. Med Chem Res. 2018;27:1043–1049. doi:10.1007/s00044-017-2126-0
Yan Q, Cao R, Yi W, Chen Z, Wen H, Ma L, Song H. Inhibitory effects of 5-benzylidene barbiturate derivatives on mushroom tyrosinase and their antibacterial activities. Eur J Med Chem. 2009;44:4235–4243. doi:10.1016/j.ejmech.2009.05.023
Egorova S, Ivanova VN, Putokhin NI. Thiophene aldehyde and its derivatives. Chem Heterocycl Comp. 1967;3:654. doi:10.1007/BF00468337
Ramesh G, Daravath S, Ganji N, Rambabu A, Venkateswarlu K. Facile synthesis, structural characterization, DNA binding, incision evaluation, antioxidant and antimicrobial activity studies of Cobalt(II), Nickle(II) and Copper(II) complexes of 3-amino-5-(4-fluorophenyl)isoxazole derivatives. J Mol Struct. 2019;1202:1–41. doi:10.1016/j.molstruc.2019.127338
Ali I, Haque A, Saleem K, Hsieh MF. Curcumin-I Knoevenagel's condensates and their Schiff's bases as anti-cancer agents: synthesis, pharmacological and simulation studies. Bioorg Med Chem. 2013;21:3808–3820. doi:10.1016/j.bmc.2013.04.018
Indumathy R, Kanthimathi M, Weyhermuller T, Nair BU. Cobalt complexes of terpyridine ligands: crystal structure and nuclease activity. Polyhedron 2008;27:3443–3450. doi:10.1016/j.poly.2008.08.003
Zhao P, Xu LC, Huang JW, Liu J, Yu HC, Zheng KC, Ji LN. Experimental and DFT studies on DNA binding and photocleavage of two cationic porphyrins: effects of the introduction of a carboxyphenyl into pyridiniumporphyrin. Spectrochim Acta A Mol Biomol Spectrosc. 2008;71:1216–1223. doi:10.1016/j.saa.2008.03.031
Hajian R, Ekhlasi E, Daneshvar R. Spectroscopic and electrochemical studies on the interaction of epirubicin with fish sperm DNA. J Chem. 2012;9:1587–1598. doi:10.1155/2012/738678
Campillos M, Kuhn M, Gavin AC, Jensen LJ, Bork P. Drug target identification using side-effect similarity. Science. 2008;321:263–266. doi:10.1126/science.1158140
Keiser MJ, Roth BL, Armbruster BN, Ernsberger P, Irwin JJ, Shoichet BK. Relating protein pharmacology by ligand chemistry. Nat Biotechnol. 2007;25:197–206. doi:10.1038/nbt1284
Ali A, Khalid M, Rehman MF, Haq S, Ali A, Tahir MN, Ashfaq M, Rasool F, Braga AAC. Efficient synthesis, SC-XRD, and theoretical studies of O-Benzenesulfonylated pyrimidines: Role of noncovalent interaction influence in their supramolecular network. ACS Omega. 2020;5:15115–15128. doi:10.1021/acsomega.0c00975
Ooretir C, Kaniskan N. Frontier orbital theory and chemical reactivity: The utility of spectroscopy and molecular orbital calculations. Recent Experimental and Computational Advances in Molecular Spectroscopy. 1993:351–367.
Anup Pandith, Young Jun Seo. Label-free sensing platform for miRNA-146a based on chromofluorogenic pyrophosphate recognition. J Inorg Biochem. 2020;203:1–41. doi:10.1016/j.jinorgbio.2019.110867
George J, Prasana JC, Muthu S, Kuruvilla TK, Savanthi S, Saji RS. Spectroscopic (FT-IR, FT-Raman) and Quantum mechani-cal study on N-(2,6 dimehyl phenyl)-2-{4-[2hydroxy-3-(2methoxyphenoxyl)propyl]piperazin-1yl}acetamide. J Mol Struct. 2018;1171:268–278. doi:10.1016/j.molstruc.2018.05.106
DOI: https://doi.org/10.15826/chimtech.2021.8.4.01
Copyright (c) 2021 S. H. Sukanya, Talavara Venkatesh, S. J. Aditya Rao, N. Shivakumara, Muthipeedika Nibin Joy

This work is licensed under a Creative Commons Attribution 4.0 International License.
Chimica Techno Acta, 2014–2025
eISSN 2411-1414
Copyright Notice







