Quantum chemical study of heterocyclic organic compounds on the corrosion inhibition
Abstract
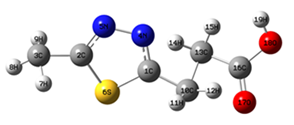
Corrosion damages all materials, necessitating replacement and inspection related expenses. Thus, the demand has increased for new corrosion inhibitor materials. The ratios of corrosion inhibition of materials are different, but organic compounds have high efficiency in aqueous corrosion inhibition for various alloys and metals. This efficiency can increase in the presence of O, N and S. The molecule provides great inhibition with the presence of both S and N atoms in the same compound. This paper investigates the 1, 3, 4-thiadiazole molecule and electronic structure of several organic compounds such as R1 and R2 which consist of different substituent groups. They were united to the ring of 1, 3, 4-thiadiazole to provide nine different derivatives. Quantum computations (density functional theory, DFT) at 6-311G++ (d, p) basis set and Becke’s three parameters hybrid (B3LYP) level were performed using Gaussian program. The purpose of this study is to determine the chemical behaviour of several heterocyclic organic compounds and to understand the process of the corrosion inhibition.
Keywords
Full Text:
PDFReferences
Potthast A, Henniges U, Banik G. Iron gall ink-induced corrosion of cellulose: aging, degradation and stabilization. Part 1: model paper studies. Cellulose. 2008;15(6):849–859. doi:10.1007/s10570-008-9237-1
Kim D-K, Muralidharan S, Ha T-H, Bae J-H, Ha Y-C, Lee H-G, Scantlebury J. Electrochemical studies on the alternating current corrosion of mild steel under cathodic protection condition in marine environments. Electrochim Acta. 2006;51(25):5259–5267. doi:10.1016/j.electacta.2006.01.054
Qadr HM. A molecular dynamics study of temperature dependence of the primary state of cascade damage processes. Russ J Non-Ferrous Metal. 2021;62(5):561–567. doi:10.3103/S1067821221050096
Qadr HM. Pressure effects on stopping power of alpha particles in argon gas. Phys Particl Nucl Lett. 2021;18(2):185–189. doi:10.1134/S1547477121020151
Sastri VS. Green corrosion inhibitors: theory and practice. John Wiley & Sons; 2012. 311 p.
Rani B, Basu BBJ. Green inhibitors for corrosion protection of metals and alloys: an overview. Int J Corros. 2012;380217. doi:10.1155/2012/380217
Sanyal B. Organic compounds as corrosion inhibitors in different environments – a review. Prog Org Coat. 1981;9(2):165–236. doi:10.1016/0033-0655(81)80009-X
Revie RW. Corrosion and corrosion control: an introduction to corrosion science and engineering. John Wiley & Sons; 2008. 512 p.
Shreir LL. Corrosion: metal/environment reactions. Newnes; 2013. 1232 p.
Schmitt G. Application of inhibitors for acid media: report prepared for the European federation of corrosion working party on inhibitors. Brit Corros J. 1984;19(4):165–176. doi:10.1179/000705984798273100
Obot I, Obi-Egbedi N. Theoretical study of benzimidazole and its derivatives and their potential activity as corrosion inhibitors. Corros Sci. 2010;52(2):657–660. doi:10.1016/j.corsci.2009.10.017
Huzinaga S, Andzelm J, Radzio-Andzelm E, Sakai Y, Tatewaki H, Klobukowski M. Gaussian basis sets for molecular calculations. Elsevier; 2012. 434 p.
Kaya S, Kaya C, Guo L, Kandemirli F, Tüzün B, Uğurlu İ, Mad-kour LH, Saraçoğlu M. Quantum chemical and molecular dynamics simulation studies on inhibition performances of some sizable and thiadiazole derivatives against corrosion of iron. J Mol Liq. 2016;219:497–504. doi:10.1016/j.molliq.2016.03.042
Amin MA. A newly synthesized glycine derivative to control uniform and pitting corrosion processes of Al induced by SCN− anions–Chemical, electrochemical and morphological studies. Corros Sci. 2010;52(10):3243–3257. doi:10.1016/j.corsci.2010.05.041
Raicheva S, Aleksiev B, Sokolova E. The effect of the chemical structure of some nitrogen- and sulphur-containing organic compounds on their corrosion inhibiting action, Corros Sci. 1993;34(2):343–350. doi:10.1016/0010-938X(93)90011-5
Growcock F. Inhibition of steel corrosion in HCl by derivatives of cinnamaldehyde: Part I. Corrosion inhibition model. Corros. 1989;45(12):1003–1007. doi:10.5006/1.3585008
Quraishi M, Sharma HK. 4-Amino-3-butyl-5-mercapto-1, 2, 4-triazole: a new corrosion inhibitor for mild steel in sulphuric acid. Mater Chem Phys. 2003;78(1):18–21. doi:10.1016/S0254-0584(02)00313-9
Tsuneda T. Density functional theory in quantum chemistry. Springer: Tokyo; 2014. doi:10.1007/978-4-431-54825-6
Qadr HM. Effect of ion irradiation on the mechanical properties of high and low copper. Atom Indones. 2020;46(1):47–51. doi:10.17146/aij.2020.923
Mamand D. Theoretical calculations and spectroscopic analysis of gaussian computational examination-NMR, FTIR, UV-Visible, MEP on 2, 4, 6-Nitrophenol. J Phys Chem Funct Mater. 2019;2(2):77–86.
Endredi G, Perczel A, Farkas O, McAllister M, Csonka G, Ladik J, Csizmadia I. Peptide models XV. The effect of basis set size increase and electron correlation on selected minima of the ab initio 2D-Ramachandran map of For-Gly-NH2 and For-l-Ala-NH2. J Mol Struct. 1997;391(1–2):15–26. doi:10.1016/S0166-1280(96)04695-7
Foresman J, Frish E. Exploring chemistry. Gaussian Inc.: Pitts-burg, USA; 1996. 304 p.
Mineva T, Russo N. Atomic Fukui indices and orbital hardnesses of adenine, thymine, uracil, guanine and cytosine from density functional computations. J Mol Struct. 2010;943(1–3):71–76. doi:10.1016/j.theochem.2009.10.023
Yang W, Mortier WJ. The use of global and local molecular parameters for the analysis of the gas-phase basicity of amines. J Am Chem Soc. 1986;108(19):5708–5711. doi:10.1021/ja00279a008
Mendez F, Galván M, Garritz A, Vela A, Gàzquez J. Local softness and chemical reactivity of maleimide: nucleophilic addi-tion. J Mol Struct. 1992;277:81–86.
doi:10.1016/0166-1280(92)87131-I
Mulliken R. Electronic population analysis on LCAO–MO molecular wave functions. II. Overlap populations, bond orders, and covalent bond energies. J Chem Phys. 1955;23(10):1841–1846. doi:10.1063/1.1740588
Kochi JK. Electron transfer and charge transfer: twin themes in unifying the mechanisms of organic and organometallic reactions. Angew Chem Int Ed English 27(10) (1988) 1227-1266. doi:10.1002/anie.198812273
Lee C, Yang W, Parr RG. Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys Rev B. 1988;37(2):785. doi:10.1103/PhysRevB.37.785
Becke AD, Johnson ER. Exchange-hole dipole moment and the dispersion interaction. J Chem Phys. 2005;122(15):154104. doi:10.1063/1.1884601
Huang Y, Rong C, Zhang R, Liu S. Evaluating frontier orbital energy and HOMO/LUMO gap with descriptors from density functional reactivity theory. J Mol Model. 2017;23(1):1–12. doi:10.1007/s00894-016-3175-x
Mamand DM, Qadr HM. Comprehensive spectroscopic and optoelectronic properties of bbl organic semiconductor. Protect Metals Phys Chem Surf. 2021;57(5):943–953. doi:10.1134/S207020512105018X
Kavitha E, Sundaraganesan N, Sebastian S. Molecular structure, vibrational spectroscopic and HOMO, LUMO studies of 4-nitroaniline by density functional method. Indian J Pure Appl Phys. 2010;10(1):53–62.
Fleming I. Frontier orbitals and organic chemical reactions. New York: Wiley; 1977. 249 p.
Lee L-H. Correlation between Lewis acid− base surface interaction components and linear solvation energy relationship solvatochromic α and β parameters. Langmuir. 1996;12(6):1681–1687. doi:10.1021/la950725u
Kim K-H, Yu H, Kang H, Kang DJ, Cho C-H, Cho H-H, Oh JH, Kim BJ. Influence of intermolecular interactions of electron donating small molecules on their molecular packing and performance in organic electronic devices. J Mater Chem A. 2013;1(46):14538–14547. doi:10.1039/C3TA13266H
Elmsellem H, Harit T, Aouniti A, Malek F, Riahi A, Chetouani A, Hammouti B. Adsorption properties and inhibition of mild steel corrosion in 1 M HCl solution by some bipyrazolic derivatives: experimental and theoretical investigations. Prot Metals Phys Chem Surf. 2015;51(5):873–884. doi:10.1134/S207020511505007X
Vandewal K, Ma Z, Bergqvist J, Tang Z, Wang E, Henriksson P, Tvingstedt K, Andersson MR, Zhang F, Inganäs O. Quantification of Quantum efficiency and energy losses in low bandgap polymer: fullerene solar cells with high open‐circuit voltage. Adv Funct Mater. 2012;22(16):3480–3490. doi:10.1002/adfm.201200608
Mamand D. Determination the band gap energy of poly ben-zimidazobenzophenanthroline and comparison between HF and DFT for three different basis sets. J Phys Chem Funct Ma-ter. 2019;2(1):32–36.
Jensen WB. Overview lecture the lewis acid-base concepts: recent results and prospects for the future. J Adhes Sci Tech-nol. 1991;5(1):1–21. doi:10.1163/156856191X00792
Kabanda MM, Obot IB, Ebenso EE. Computational study of some amino acid derivatives as potential corrosion inhibitors for different metal surfaces and in different media. Int J Elec-trochem Sci. 2013;8:10839–10850.
Kaya S, Kaya C. A new equation for calculation of chemical hardness of groups and molecules. Mol Phys. 2015;113(11):1311–1319. doi:10.1080/00268976.2014.991771
Chakraborty T, Hens A, Kulashrestha S, Murmu NC, Banerjee P. Calculation of diffusion coefficient of long chain molecules using molecular dynamics. Phys E Low Dimens Syst Nanostruct. 2015;69:371–377. doi:10.1016/j.physe.2015.01.008
Saha SK, Hens A, RoyChowdhury A, Lohar AK, Murmu N, Banerjee P. Molecular dynamics and density functional theory study on corrosion inhibitory action of three substituted pyrazine derivatives on steel surface. Can Chem Trans. 2014;2(4):489–503. doi:10.13179/canchemtrans.2014.02.04.0137
Özcan M, Dehri İ, Erbil M. Organic sulphur-containing compounds as corrosion inhibitors for mild steel in acidic media: correlation between inhibition efficiency and chemical structure. Appl Surf Sci. 2004;236(1–4):155–164. doi:10.1016/j.apsusc.2004.04.017
Bereket G, Hür E, Öğretir C. Quantum chemical studies on some imidazole derivatives as corrosion inhibitors for iron in acidic medium. J Mol Struct. 2002;578(1–3):79–88. doi:10.1016/S0166-1280(01)00684-4
Obot I, Obi-Egbedi N. Adsorption properties and inhibition of mild steel corrosion in sulphuric acid solution by ketoconazole: experimental and theoretical investigation. Corros Sci. 2010;52(1):198–204. doi:10.1016/j.corsci.2009.09.002
Saha SK, Hens A, Murmu NC, Banerjee P. A comparative density functional theory and molecular dynamics simulation studies of the corrosion inhibitory action of two novel N-heterocyclic organic compounds along with a few others over steel surface. J Mol Liq. 2016;215:486–495. doi:10.1016/j.molliq.2016.01.024
Cossi M, Barone V, Cammi R, Tomasi J. Ab initio study of solv-ated molecules: a new implementation of the polarizable continuum model. Chem Phys Lett. 1996;255(4–6):327–335. doi:10.1016/0009-2614(96)00349-1
Foresman JB, Keith TA, Wiberg KB, Snoonian J, Frisch MJ. Influence of cavity shape, truncation of electrostatics, and electron correlation on ab initio reaction field calculations. J Phys Chem. 1996;100(40):16098–16104. doi:10.1021/jp960488j
Hasanov R, Sadıkoğlu M, Bilgiç S. Electrochemical and quantum chemical studies of some Schiff bases on the corrosion of steel in H2SO4 solution. Appl Surf Sci. 2007;253(8):3913–3921. doi:10.1016/j.apsusc.2006.08.025
Pearson RG. Absolute electronegativity and hardness correlated with molecular orbital theory. Proc Nat Acad Sci. 1986;83(22):8440–8441. doi:10.1073/pnas.83.22.8440
Qadr HM, Mamand DM. Molecular structure and density functional theory investigation corrosion inhibitors of some oxadiazoles. J Bio Tribo Corros. 2021;7(4):1–8. doi:10.1007/s40735-021-00566-9
Gómez B, Likhanova NV, Domínguez-Aguilar MA, Martínez-Palou R, Vela A., Gazquez JL. Quantum chemical study of the inhibitive properties of 2-pyridylazoles. J Phys Chem B. 2006;110(18):8928–8934. doi:10.1021/jp057143y
Qadr HM, Hamad AM. Using of stopping and range of ions in Matter code to study of radiation damage in materials. RENSIT. 2020;12(4):451–456. doi:10.17725/rensit.2020.12.451
DOI: https://doi.org/10.15826/chimtech.2022.9.2.03
Copyright (c) 2021 Dyari Mustafa Mamand, Awat Hamad Awla, Twana Mohammed Kak Anwer, Hiwa Mohammad Qadr

This work is licensed under a Creative Commons Attribution 4.0 International License.
Chimica Techno Acta, 2014–2025
eISSN 2411-1414
Copyright Notice






