Features of -C-C- coupling of quinoxaline-2-one with ethyl acetoacetate under acid catalysis
Abstract
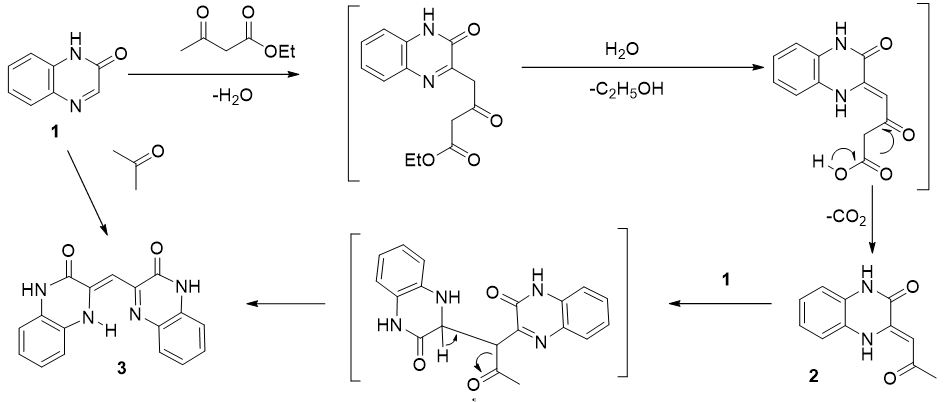
Quinoxalin-2-one (1) reacts with ethyl acetoacetate in trifluoroacetic acid (TFA) to form 3-(2-oxopropylideno)-3,4-dihydroquinoxaline-2-one (2) and 3-(3-oxo-3,4- dihydroquinoxaline-2-(1H)-ylidene)methylquinoxaline-2-(1H)-one (3). The reaction product 3 was also obtained by heating the compound 1 with acetone in the presence of TFA.
Keywords
Full Text:
PDFReferences
Barlin GB. The Chemistry of Heterocyclic Compounds: The Pyrazines. New York: Wiley-VCH; 1982. 687 p. doi:10.1002/9780470187173
Cheeseman GWN, Cookson RF. The Chemistry of Heterocyclic Compounds: The Condensed Pyrazines. New York: Wiley-VCH; 1979. 843 p.
Mashkovsky MD. Lekarstvennyye sredstva [Medicines]. Moscow: Nauka; 1993. 347 p. Russian.
Sakato G, Makino K, Kurasawa Y. Recent progress in the quinoxaline chemistry. Synthesis and biological activity. Heterocycles. 1988;27(10):2481–515. doi:10.3987/REV-88-397
Chupakhin ON, Charushin VN, Klyuev NA, Rezvukhin AI, Semion VA. Cyclization of N-alkylazinium cations with bisnucleophiles. 3.endo adducts in the reaction of quinoxalium salts with β-diketones and their x-ray diffraction analysis. Chem Het Comp. 1981;17:1046–1052. doi:10.1007/BF00503539
Schmidt A, Guetlein J-P, Langer P. Synthesis of 6-alkylidene-2,3-benzo-1,4-diaza-7-oxabicyclo[4.3.0]non-2-enes by cyclization of 1,3-bis(silyl enol ethers) with quinoxalines. Tetr Let. 2007;48:2067–2069. doi:10.1016/j.tetlet.2007.01.147
Azev YuA, Kodess MI, Ezhikova MA, Gibor AM, Baranov VI, Ermakova OS, Bakulev VA. New opportunities for the synthesis of quinoxaline-substituted heterocyclic and aryl moieties. Pharm Chem J. 2013;9:498–502. doi:10.1007/s11094-013-0989-z
Azev YuA, Kodess MI, Ezhikova MA, Ermakova OS, Berseneva VS, Bakulev VA. Reactions of quinoxaline-2-one with β-diketones: a new approach to 6a,7-dihydro-5H-pyrido[1,2-a]quinoxaline-6,8-diones. Mend Comm. 2017;1:97–98. doi:10.1016/j.mencom.2017.01.032
Azev YuA, Ermakova OS, Berseneva VS, Kodess MI, Ezhikova MA, Ganebnykh IN. Synthesis of the 6-oxidopyrido[1,2-a]quinoxalinium derivatives from quinoxaline-2-one and aldehydes – new examples of domino reactions. Mend Comm. 2017;27:637–639. doi: 10.1016/j.mencom.2017.11.034
Azev YuA, Koptyaeva OS, Tsmokalyuk AN, Pospelova TA, Bakulev VA. Benzaldehyde phenylhydrazones as C-nucleophiles for functionalization of quinoxaline-2-one. Unusual transformations of indole-3-carbaldehyde phenylhydrazones. AIP Conf Proc. 2020;2280:0018799. doi:10.1063/5.0018799
Tennant G. Heterocyclic N-oxides. Part II. Nucleophilic reactions of 1,2-Dihydro-2-oxoquinoxaline 4-oxide. J Chem Soc. 1964;1982–1986.
DOI: https://doi.org/10.15826/chimtech.2022.9.1.03
Copyright (c) 2021 Y.A. Azev, O.S. Koptyaeva, A.A. Mkrtchan, T.A. Pospelova

This work is licensed under a Creative Commons Attribution 4.0 International License.
Chimica Techno Acta, 2014–2025
eISSN 2411-1414
Copyright Notice






