New naphtho[1,8-ef]perimidines: synthesis, fluorescence studies and application for detection of nitroanalytes
Abstract
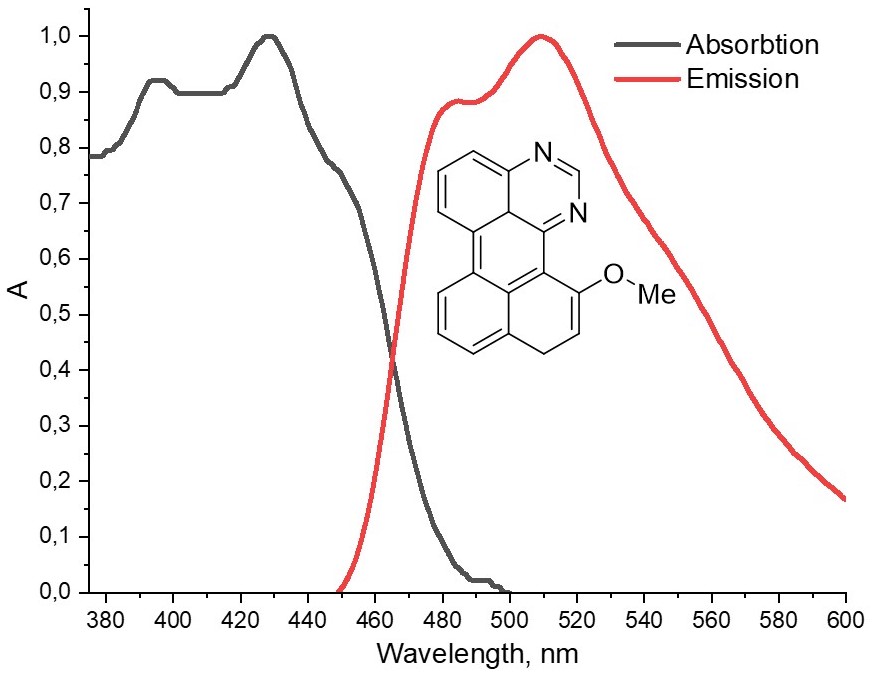
A rational approach to the synthesis of substituted naphtho[1,8-ef]perimidines based on SNH methodology and cyclization reaction in the series of condensed azines with naphthalene substituents was presented. Photophysical properties of the obtained fluorophores were studied, in particular, green fluorescence in the 485–536 nm range with quantum yield up to 32.4% was detected. HOMO-LUMO energy values and distributions for the new compounds were calculated by the DFT method in comparison with nitroanalytes and perylene. Based on the data obtained, as well as on the results of fluorescence titration, the possibility of using the new diazaperylenes as potential chemosensors for the visual detection of nitro-containing explosives was shown.
Keywords
Full Text:
PDFReferences
Zyryanov GV, Kopchuk DS, Kovalev IS, Nosova EV, Rusinov VL, Chupakhin ON. Chemosensors for detection of nitroaromatic compounds (explosives). Russ Chem Rev. 2014;83(9):783–819. doi:10.1070/RC2014v083n09ABEH004467
Lee JH, Rock JC, Schlautman MA, Carraway ER. Characteristics of key intermediates generated in uncatalyzed bis(2,4-dinitrophenyl) oxalate (DNPO) chemiluminescence reactions. J Chem Soc Perkin Trans. 2002;2:1653–7. doi:10.1039/b206367k
Pandey S, Fletcher KA, Powell JR, McHale MER, Kauppila A-SM, Acree, Jr. WE, Fetzer JC, Dai W, Harvey RG. Spectrochim. Acta, Part A. 1997;53:165–72. doi:10.1007/978-0-387-46312-4_8
Zhang P-F, Zeng J-C, Zhuang F-D, Zhao K-X, Sun Z-H, Yao Z-F, Lu Y, Wang X-Y, Wang J-Y, Pei J. Parent B2N2-perylenes with different BN orientations. Angew Chem Int Ed. 2021;60:23313–9. doi:10.1002/anie.202108519
Cui X, Zhao J, Yang P, Sun J. Zinc(II) tetraphenyltetrabenzoporphyrin complex as triplet photosensitizer for triplet–triplet annihilation upconversion. Chem. Commun. 2013;49:10221–3. doi:10.1039/c3cc45843a
Murakami K, Yamada S, Kaneda T, Itami K. C–H Functionalization of Azines. Chem Rev. 2017;117:9302–32. doi:10.1021/acs.chemrev.7b00021
Abengózar A, Valencia I, Otárola GG, Sucunza D, García-García P, Pérez-Redondo A, Mendicuti F, Vaquero JJ. Expanding the BN-embedded PAH family: 4a-aza-12a-borachrysene. Chem Commun. 2020;56:3669–72. doi:10.1039/C9CC09998K
Hirono A, Sakai H, Hasobe T. Synthesis and electrochemical and photophysical properties of azaterrylene derivatives. Chem. Asian J. 2019;14:1754–62. doi:10.1002/asia.201801410.
Harwell JR, Glackin JME, Davis NJLK, Gillanders RN, Credgington D, Turnbull GA, Samuel IDW. Sensing of explosive vapor by hybrid perovskites: effect of dimensionality. APL Mater. 2020;8:071106. doi:10.1063/5.0011229
Neese F. The ORCA program system. WIREs Comput Mol Sci. 2012;2:73–78. doi:10.1002/wcms.81
Porrès L, Holland A, Palsson L-O, Monkman AP, Kemp C, Beeby A. Absolute measurements of photoluminescence quantum yields of solutions using an integrating sphere. J Fluoresc. 2006;16:267–73.doi:10.1007/s10895-005-0054-8
Utepova IA, Nemytov AI, Ishkhanian VA, Chupakhin ON, Charushin VN. Metal-free C–H/C–H coupling of 1,3-diazines and 1,2,4-triazines with 2-naphthols facilitated by Brønsted acids. Tetrahedron. 2020;76:131391. doi:10.1016/j.tet.2020.131391
Gryko DT, Piechowska J, Gaze-zowski M. Strongly emitting fluorophores based on 1-azaperylene scaffold. J Org Chem. 2010;75:1297–30. doi:10.1021/jo902443s
Krishnan R, Binkley JS, Seeger R, Pople JA. Self‐consistent molecular orbital methods. XX. A basis set for correlated wave functions. J Chem Phys. 1980;72:650–4. doi:10.1063/1.438955
McLean AD, Chandler J. GS. Contracted Gaussian basis sets for molecular calculations. I. Second row atoms, Z=11–18. Chem Phys. 1980;72:5639–48. doi:10.1063/1.438980
Clark T, Chandrasekhar J, Schleyer PvR. Efficient diffuse function-augmented basis sets for anion calculations. III. The 3-21+G basis set for first-row elements, Li–F. J Comp Chem. 1983;4:294–301. doi:10.1002/jcc.540040303
Frisch MJ, Pople JA, Binkley JS. Self‐consistent molecular orbital methods 25. Supplementary functions for Gaussian basis sets. J Chem Phys. 1984;80:3265–9. doi:10.1063/1.447079
Mohamad M, Ahmed R, Shaari A, Goumri‑Said S. Structure‑dependent optoelectronic properties of perylene, di‑indenoperylene (DIP) isolated molecule and DIP molecular crystal. Chem Cent J. 2017;11:125. doi:10.1186/s13065-017-0352-7
Shrivastava A, Gupta V. Methods for the determination of limit of detection and limit of quantitation of the analytical methods. Chronicles Young Sci. 2011;2:21. doi:10.4103/2229-5186.79345
DOI: https://doi.org/10.15826/chimtech.2021.8.4.16
Copyright (c) 2021 Igor L. Nikonov, Leila K. Sadieva, Мaria I. Savchuk, Еkaterina S. Starnovskaya, Dmitry S. Kopchuk, Igor S. Kovalev, Grigory А. Кim, Oleg N. Chupakhin

This work is licensed under a Creative Commons Attribution 4.0 International License.
Chimica Techno Acta, 2014–2025
eISSN 2411-1414
Copyright Notice







