New 2,5-bis(2-ethylhexyl)pyrrolo[3,4-c]pyrrole-1,4(2H,5H)-dione-2,2’-bipyridine-based co-polymer, synthesis, photophysical properties and response to metal cations
Abstract
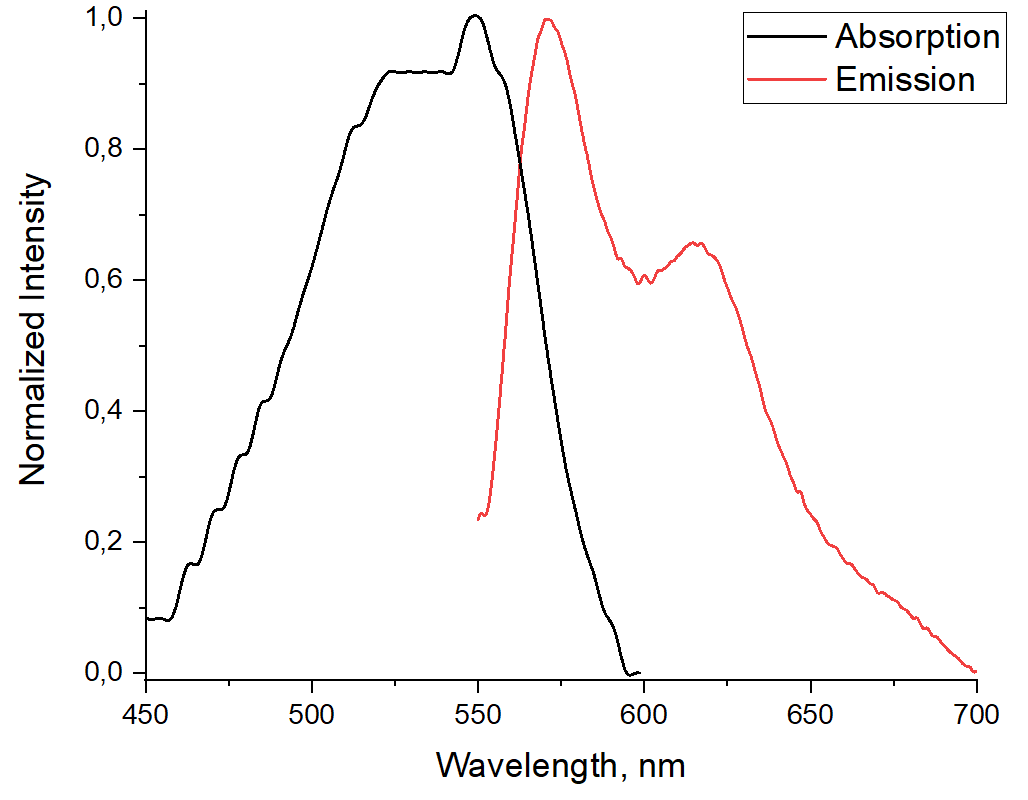
A new co-polymer based on fragments of 2-(2-pyridyl)monoazatriphenylene and 2,5-bis (2-ethylhexyl)-3,6-di(thiophen-2-yl)pyrrolo[3,4-c]pyrrole-1,4(2H,5H)-dione was prepared by using the Sonogashira reaction. The photophysical properties of the polymer were studied. The presence of a strong bathochromic shift of the absorption and emission maxima in comparison with the previously described monomer units is shown. The polymer exhibits an intense “turn-off” response toward Cu2+ cations.
Keywords
Full Text:
PDFReferences
Liu J, Lam JWY, Tang BZ. Acetylenic polymers: syntheses, structures, and functions. Chem Rev. 2009;109(11):5799–5867. doi:10.1021/cr900149d
Wang T, Zhang N, Baic W, Bao Y. Fluorescent chemosensors based on conjugated polymers with N-heterocyclic moieties: two decades of progress. Polym Chem. 2020;11:3095–3114. doi:10.1039/d0py00336k
Wang B, Wasielewski MR. Design and synthesis of metal ion-recognition-induced conjugated polymers: an approach to metal ion sensory materials. J Am Chem Soc. 1997;119(1):12–21. doi:10.1021/ja962229d
Xing CF. Shi ZQ, Yu MH, Wang S. Cationic conjugated poly-electrolyte-based fluorometric detection of copper(II) ions in aqueous solution. Polymer. 2008;49(11):2698–2703. doi:10.1016/j.polymer.2008.04
Li C, Zhou C, Zheng H, Yin X, Zuo Z, Liu H, Li Y. Synthesis of a novel poly(para-phenylene ethynylene) for highly selective and sensitive sensing mercury (II) ions. J Polym Sci Part A: Polym Chem. 2008;46:1998–2007. doi:10.1002/pola.22534
Ha JS, Kim KH, Choi DH. 2,5-Bis(2-octyldodecyl)pyrrolo[3,4-c]pyrrole-1,4-(2H,5H)-dione-based donor–acceptor alternating copolymer bearing 5,5′-di(thiophen-2-yl)-2,2′-biselenophene exhibiting 1.5 cm2·V–1·s–1 hole mobility in thin-film transistors. J Am Chem Soc. 2011;133(27):10364–10367. doi:10.1021/ja203189h
Lee TW, Lee DH, Shin J, Cho MJ, Choi DH. π-Conjugated pol-ymers derived from 2,5-bis(2-decyltetradecyl)-3,6-di(selenophen-2-yl)pyrrolo[3,4-c]pyrrole-1,4(2H,5H)-dione for high-performance thin film transistors. Polym Chem. 2015;6(10):1777–1785. doi:10.1039/C4PY01536C
Lee JH, Park GE, Choi S, Lee DH, Um HA, Shin J, Cho MJ, Choi DH. Effect of the thiophene and selenophene moiety in regular terpolymers on the performance of thin film transistors and polymer solar cells. Polymer. 2016;94:43–52. doi:10.1016/j.polymer.2016.04.037
Wei C, Zhang W, Zhou Y, Huang J, Li D, Wang Q, Yu G. High-performance ternary π-conjugated copolymers containing diarylethylene units: synthesis, properties, and study of substituent effects on molecular aggregation and charge transport characteristics. J Mater Chem C. 2019;7:362–370. doi:10.1039/C8TC04940H
Makoto F, Kunihiko K, Hiroshi Y, Keiichi M. Evaluation of new organic pigments as laser-active media for a solid-state dye laser. Dyes and Pigments. 2004;63(2):115–125. doi:10.1016/j.dyepig.2004.02.002
Ftouni H, Bolze F, Nicoud JN. Water-soluble diketo-pyrrolopyrrole derivatives for two-photon excited fluorescence microscopy. Dyes Pigm. 2013;97(1):77–83. doi:10.1016/j.dyepig.2012.11.028
Nie K, Dong B, Shi H, Chao L, Duan X, Jiang XF, Liu Z, Liang B. N-alkylated diketopyrrolopyrrole-based ratiometric/fluorescent probes for Cu2+ detection via radical process. Dyes Pigments. 2019;160:814–822. doi:10.1016/j.dyepig.2018.09.027
Nie K, Dong B, Shi H, Liu Z, Liang B. Thienyl diketo-pyrrolopyrrole as a robust sensing platform for multiple ions and its application in molecular logic system. Sensor Actuator B Chem. 2017;244:849–853. doi:10.1016/j.snb.2017.01.037
Grzybowski M, Gryko DT. Diketopyrrolopyrroles: synthesis, reactivity, and optical properties. Adv Optical Mater. 2015;3(3):280–320. doi:10.1002/adom.201400559
Jianga X, Wanga L, Tanga H, Caoa D, Chen W. Diketo-pyrrolopyrrole: an emerging phototherapy agent in fighting cancer. Dyes Pigments. 2020;181:108599–609. doi:10.1016/j.dyepig.2020.108599
Jin X, Xing X, Deng Q, Qing W, Liu Z, Huang Y. Molecular engineering of diketopyrrolopyrrole-conjugated polymer nanoparticles by chalcogenide variation for photoacoustic imaging guided photothermal therapy. J Mater Chem B. 2021;9:3153–3160. doi:10.1039/D1TB00193K
Wang P, Li H, Gu C, Dong H, Xua Z, Fu H. Air-stable ambipolar organic field-effect transistors based on naphtha-lenediimide–diketopyrrolopyrrole copolymers. RSC Adv. 2015;5:19520–19527. doi:10.1039/C5RA00391A
Khasanov AF, Kopchuk DS, Krinochkin AP, Kovalev IS, Taniya OS, Suvorova AI, Zyryanov GV, Rusinov VL, Chupakhin ON. 2,7-diehtynyl-10-(pyridin-2-yl)-12,13-dihydro-11H-dibenzo[f,h]cyclopenta[c]quinoline as potential monomer for creating polymers for different tasks. AIP Conf Proc. 2020;2280:040021–040024. doi:10.1063/5.0018783
Yang JS, Swager TM. Fluorescent Porous Polymer Films as TNT Chemosensors: Electronic and Structural Effects. J Am Chem Soc. 1998;120:11864–11873. doi:10.1021/ja982293q
Kopchuk DS, Khasanov AF, Kovalev IS, Zyryanov GV, Kim GA, Nikonov IL, Rusinov VL, Chupakhin ON. The extension of conjugated system in pyridyl-substituted monoazatriphenylenes for the tuning of photophysical properties. Chem Heterocycl Comp. 2014;50(6):871–879. doi:10.1007/s10593-014-1541-0
Chakali M, Mandal H, Venkatesan M, Dyaga B, Rao VJ, Bangal PR. Charge separation and singlet fission in covalently linked diketopyrrolopyrrole derivatives and triphenylamine triad in solution. J Photochem Photobiol A: Chem. 2021;406:113017–113028. doi:10.1016/j.jphotochem.2020.113017
DOI: https://doi.org/10.15826/chimtech.2021.8.4.17
Copyright (c) 2021 Alexey P. Krinochkin, Мaria I. Savchuk, Еkaterina S. Starnovskaya, Igor L. Nikonov, Artem V. Baklykov, Ekaterina A. Kudryashova, Svetlana S. Rybakova, Evgeny D. Ladin, Dmitry S. Kopchuk, Zhuo Wang, Oleg N. Chupakhin

This work is licensed under a Creative Commons Attribution 4.0 International License.
Chimica Techno Acta, 2014–2025
eISSN 2411-1414
Copyright Notice







