A method of mild deoxydichlorination of aldehydes catalyzed by Triphenylphosphine oxide
Abstract
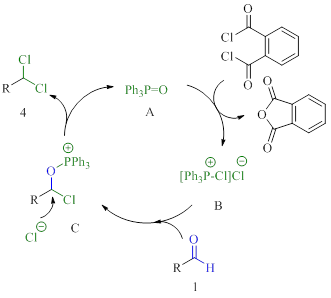
The catalytic system of triphenylphosphine oxide and phthaloyl dichloride catalysing conversion of aldehydes to 1,1-dichlorides is reported. The reaction proceeds via a P (V) catalysis manifold in which triphenylphosphine oxide turnover is achieved using phthaloyl dichloride as a consumable reagent. The application of the developed method on substrates of different structures was demonstrated. We showed the use of unsaturated compounds, including aromatics with and without electron donating / withdrawing groups, as well as saturated aliphatic compounds. The possibility of using the developed method on a gram scale was also demonstrated with the deoxydichlorination reaction of 0.03 mol of benzaldehyde catalyzed by triphenylphosphine oxide as an example. The proposed method may be of interest for the production of different herbicides, insecticides and fungicides for the agricultural industry.
Keywords
Full Text:
PDFReferences
Li J, Huang ChYu, Li ChJ. Deoxygenative functionalizations of aldehydes, ketones and carboxylic acids. Angew Chem Int Ed Engl. 2021:202112770. doi:10.1002/anie.202112770
Beddoe RH, Sneddon HF, Denton RM. The catalytic Mitsunobu reaction: a critical analysis of the current state-of-the-art. Org Biomol Chem. 2018;(42):7774–7781. doi:10.1039/C8OB01929K
Roya R, Saha S. Scope and advances in the catalytic propargylic substitution reaction. RSC Adv. 2018;(8):31129–31193. doi:10.1039/C8RA04481C
Hakim Siddiki SMA, Rashed N, Ali A, Toyao T, Hirunsit P, Ehara M, Shimizu K. Lewis acid catalysis of Nb2O5 for reactions of carboxylic acid derivatives in the presence of basic inhibitors. Chem Cat Chem. 2019;(11):383–396. doi:10.1002/cctc.201801239
Jovanovic MD, Petkovic MR, Savic VM. Polycyclic Compounds from Allenes via Palladium-Mediated intramolecular carbopalladation/nucleophilic substitution cascade processes. Synthesis 2021;(53):1035–1045. doi:10.1055/s-0040-1705994
Huy PH. Lewis Base catalysis promoted nucleophilic substitutions – recent advances and future directions. Eur J Org Chem. 2020;(1):10–27. doi:10.1002/ejoc.201901495
Beddoe RH, Andrews KG, Magné V, Cuthbertson JD, Saska J, Shannon-Little AL, Shanahan SE, Sneddon HF, Denton RM. Redox-neutral organocatalytic Mitsunobu reactions. Sci. 2019;365(6556)910–914. doi:10.1126/science.aax3353
Shipilovskikh SA, Rubtsov AE. Dehydration of oxime to nitriles. AIP Conf Proc. 2019;2063:030019. doi:10.1063/1.5087327
Huy PH, Hauch T, Filbrich I. Lewis Base catalyzed nucleophilic substitutions of alcohols. Synlett. 2016;27(19):2631–2636. doi:10.1055/s-0036-1588633
Kohlmeyer C, Schäfer A, Huy PH., Hilt G. Formamide-catalyzed nucleophilic substitutions: mechanistic insight and rationalization of catalytic. ACS Catal. 2020;10(19):11567–11577. doi:10.1021/acscatal.0c03348
Shipilovskikh SA, Vaganov VY, Denisova EI, Rubtsov AE, Malkov AV. Dehydration of amides to nitriles under conditions of a catalytic appel reaction. Org Lett. 2018;20(3):728–731. doi:10.1021/acs.orglett.7b03862
Huy PH, Mbouhom C. Formamide catalyzed activation of carboxylic acids – versatile and cost-efficient amidation and esterification. Chem Sci. 2019;10:7399–7406. doi:10.1039/C9SC02126D
Motsch S, Schütz C, Huy PH. Systematic evaluation of sulfoxides as catalysts in nucleophilic substitutions of alcohols. Eur J Org Chem. 2018:4541–4547. doi:10.1002/ejoc.201800907
Huy PH, Filbrich I. A general catalytic method for highly cost- and atom-efficient nucleophilic substitutions. Chem Eur J. 2018;24:7410. doi:10.1002/chem.201800588
Fukazawa Y, Vaganov VY, Shipilovskikh SA, Rubtsov AE, Malkov AV. Stereoselective synthesis of atropisomeric bipyridine N,N′-dioxides by oxidative coupling. Org Lett. 2019;21(12):4798–4802. doi:10.1021/acs.orglett.9b01687
Concellón JM, Rodríguez-Solla H, Díaz P, Llavona R. The first sequential reaction promoted by manganese: complete stereoselective synthesis of (E)-α,β-unsaturated esters from 2,2-dichloroesters and aldehydes. J Org Chem. 2007;72:4396. doi:10.1021/jo070209w
Concellón JM, Rodríguez-Solla H, de Amo V, Díaz P. Stereoselective olefination reactions promoted by rieke manganese. Synth. 2009;15:2634–2645. doi:10.1055/s-0029-1216880
Oudeyer S, Leonel E, Paugam JP, Nédélec JY. Formation of epoxides and N-arylaziridines via a simple Mg-Barbier reaction in DMF. Tetrahedron. 2014;70:919–923. doi:10.1016/j.tet.2013.12.016
Zhou YY, Uyeda C. Reductive cyclopropanations catalyzed by dinuclear nickel complexes. Angew Chem Int Ed. 2016;55:3171–3175. doi:10.1002/anie.201511271
Durán-Peña MJ, Flores-Giubi ME, Botubol-Ares JM, Har-wood LM, Collado IG, MacÍas-Sánchez AJ, Hernández-Galán R. Chemoselective and stereoselective lithium carbenoid mediated cyclopropanation of acyclic allylic alcohols. Org Biomol Chem. 2016;14(9):2731–2741. doi:10.1039/c5ob02617b
Barrero AF, Herrador MM, Del Moral JFQ, Arteaga P, Akssira M, El Hanbali F, Arteaga JF, Diéguez HR, Sánchez EM. Couplings of benzylic halides mediated by titanocene chloride: Synthesis of bibenzyl derivatives. J Org Chem. 2007;72(6):2251–2254. doi:10.1021/jo062492p
Eisch JJ, Qian Y, Rheingold AL. Nickel(II)-carbene intermediates in reactions of geminal dihaloalkanes with nickel(0) reagents and the corresponding carbene capture as the phosphonium ylide. Eur J Inorg Chem. 2007;(11):1576–1584. doi:10.1002/ejic.200601106
Giannerini M, Fañanas-Mastral M, Feringa BL. Z-selective copper-catalyzed asymmetric allylic alkylation with grignard reagents. J Am Chem Soc. 2012;134(9):4108–4111. doi:10.1021/ja300743t
Li H, Müller D, Guénée L, Alexakis A. Copper-catalyzed enantioselective synthesis of axially chiral allenes. Org Lett. 2012;14(23):5880–5883. doi:10.1021/ol302790e
Li H, Grassi D, Guénée L, Bürgi T, Alexakis A. Copper-catalyzed propargylic substitution of dichloro substrates: Enantioselective synthesis of trisubstituted allenes and formation of propargylic quaternary stereogenic centers. Chem Eur J. 2014;20(50):16694–706. doi:10.1002/chem.201404668
Brześkiewicz J, Loska R, Makosza M. α-Chlorobenzylation of nitroarenes via vicarious nucleophilic substitution with benzylidene dichloride: Umpolung of the friedel-crafts reaction. J Org Chem. 2018;83(15):8499–8508. doi:10.1021/acs.joc.8b01091
Nilewski C, Carreira EM. Recent advances in the total synthesis of chlorosulfolipids. Eur J Org Chem. 2012;(9):1685–1698. doi:10.1002/ejoc.201101525
Chung WJ, Vanderwal CD. Stereoselective halogenation in natural product synthesis. Angew Chem Int Ed. 2016;55:4396–4434. doi:10.1002/anie.201506388
Murawska A, Migdal P, Roman A. Effects of plant protection products on biochemical markers in honey bees. Agriculture. 2021;11(7):648. doi:10.3390/agriculture11070648
Syafrudin M, Kristanti RA, Yuniarto A, Hadibarata T, Rhee J, Alonazi WA, Algarni TS, Almarri AH, Al-Mohaimeed AM. Pesticides in drinking water - a review. Int J Environ Res Public Health. 2021;18(2):468. doi:10.3390/ijerph18020468
Tudi M, Ruan HD, Wang L, Lyu J, Sadler R, Connell D, Chu C, Phung DT. Agriculture development, pesticide application and its impact on the environment. Int J Environ Res Public Health. 2021;18(3):1112. doi:10.3390/ijerph18031112
Hirayama T, Okaniwa M, Banno H, Kakei H, Ohashi A, Iwai K, Ohori M, Mori K, Gotou M, Kawamoto T, Yokota A, Ishi-kawa T. Synthetic studies on centromere-associated protein-e (cenp-e) inhibitors: 2. application of electrostatic potential map (EPM) and structure-based modeling to imid-azo[1,2-a]pyridine derivatives as antitumor agents. J Med Chem. 2015;58:8036–8053. doi:10.1021/acs.jmedchem.5b00836
Barma DK, Kundu A, Bandyopadhyay A, Kundu A, Sangras B, Briot A, Mioskowski C, Falck J.R. A highly stereospecific synthesis of (E)-α,β-unsaturated esters. Tetrahedron Lett. 2004;45:59175920. doi:10.1016/j.tetlet.2004.05.113
Oudeyer S, Léonel E, Paugam J. P., Nédélec J.-Y. Epoxide formation by indirect electroreductive coupling between aldehydes or ketones and activated gemdichloro compounds. Synthesis. 2004;3:389–400. doi:10.1055/s-2004-815915
Cahard E, Schoenebeck F, Garnier J, Cutulic SPY, Zhou S, Murphy JA. Electron transfer to benzenes by photoactivated neutral organic electron donor molecules. 2012;51:3673–3676. doi:10.1002/anie.201200084
Roth HD, Sauers RR, Theisen KJ, Neshchadin D, Gescheidt G. Radical cations of disubstituted cyclopropanes: stereoelectronic effects on hyperfine coupling. J Phys Org Chem. 2014;27:218–225. doi:10.1002/poc.3269
Cao H, Wang Q. E-Stilbene derivatives synthesized by ste-reoselective reductive coupling of benzylic gemdibromide promoted by Cu/polyamine. Tetrahedron Letters. 2017;58:2703–2706. doi:10.1016/j.tetlet.2017.05.072
Povie G, Segawa Y, Nishihara T, Miyauchi Y, Itami K. Synthesis and size-dependent properties of [12], [16], and [24] carbon nanobelts. J Am Chem Soc. 2018;140:10054–10059. doi:10.1021/jacs.8b06842
Povie G, Segawa Y, Nishihara T, Miyauchi Y, Itami K. Syn-thesis of a carbon nanobelt. Sci. 2017;356:172-175. doi:10.1126/science.aam8158
Lin Z, Yu D, Zhang Y. Propargylic amines constructed via copper-catalyzed three-component coupling of terminal alkynes, benzal halides and amines. Tetrahedron Letters. 2011;52:4967–4970. doi:10.1016/j.tetlet.2011.07.099
Shioe K, Ishikura S, Horino Y, Abe H. Facile Preparation of Dehydrodigallic Acid and Its Derivative for the Synthesis of Ellagitannins. Chem Pharm Bull. 2013;61:1308–1314. doi:10.1248/cpb.c13-00458
Sturala J, Etherington MK, Bismillah AN, Higginbotham HF, Trewby W, Aguilar JA, Bromley EHC, Avestro A-J, Monkman AP, McGonigal PR. Excited-State Aromatic Interactions in the Aggregation-Induced Emission of Molecular Rotors. J Am Chem Soc. 2017;139:17882–17889. doi:10.1021/jacs.7b08570
Chana CY, Barnard PJ. Rhenium complexes of bidentate, bis-bidentate and tridentate N-heterocyclic carbene ligands. Dalton Transactions. 2015;44:19126–19140. doi:10.1039/C5DT03295D
Wang L, Moss RA, Krogh-Jespersen K. Hammett Analyses of Halocarbene–Halocarbanion Equilibria. Org Lett. 2013;15:2014–2017. doi:10.1021/ol400698y
Lee J, Yoon S, Chang R. Chlorosulfolipid (Danicalipin A) membrane structure: hybrid molecular dynamics simulation studies. J Phys Chem Lett. 2021;19:4537–4542. doi:10.1021/acs.jpclett.1c01026
Gropp C, Fischer S, Husch T, Trapp N, Carreira EM, Diederich F. Molecular recognition and cocrystallization of methylated and halogenated fragments of danicalipin a by enantiopure alleno-acetylenic cage receptors. J Am Chem Soc. 2020;142:4749–4755. doi:10.1021/jacs.9b13217
Moss FR, Cabrera GE, McKenna GM, Salerno GJ, Shuken SR, Landry ML, Weiss TM, Burns NZ, Boxer SG. Halogenation-dependent effects of the chlorosulfolipids of ochromonas danica on lipid bilayers. ACS Chem Biol. 2020;11:2986–2995. doi:10.1021/acschembio.0c00624
Boshkow J, Fischer S, Bailey AM, Wolfrum S, Carreira EM. Stereochemistry and biological activity of chlorinated lipids: a study of danicalipin A and selected diastereomers. Chem Sci. 2017;8:6904–6910. doi:10.1039/C7SC03124F
Takeda T, Endo Y, Reddy ACS, Sasaki R, Fujiwara T. Transformation of ketones into 1-chloro and 1,1-dichloro-1-alkenes by means of a polychloromethane-titanocene(II) system. Tetrahedron. 1999;55:2475. doi:10.1016/S0040-4020(99)00021-6
Takeda T, Sasaki R, Fujiwara T. Carbonyl Olefination by Means of a gem-Dichloride−Cp2Ti[P(OEt)3]2 System. J Org Chem. 1998;63(21):7286–7288. doi:10.1021/jo980724h
Sindra HC, Santos CVP, Mattos MCS. Trihaloisocyanuric acids: useful reagents for conversion of benzaldehydes into benzylidene dihalides under appel conditions. Lett Org Chem. 2020;17(8):590–595. doi:10.2174/1570178617666200121110618
An J, Tang X, Moore J, Lewis W, Denton RM. Phosphorus(V)-catalyzed dichlorination reactions of aldehydes. Tetrahedron. 2013;69:8769–8776. doi:10.1016/j.tet.2013.07.100
Huy PH. Formamide catalysis facilitates the transformation of aldehydes into geminal dichlorides. Synthesis. 2019;51(12):2474–2483. doi:10.1055/s-0037-1611798
Gorbunova IA, Shipilovskikh DA, Shipilovskikh SA. Deoxydichlorination of aldehydes catalyzed by Diphenyl sulfoxide. Chim Techno Acta. 2021;8(4):20218408. doi:10.15826/chimtech.2021.8.4.08
Burton KI, Elser I, Waked AE, Wagener T, Andrews RJ, Glo-rius F, Stephan DW. Bipyridinium and phenanthrolinium dications for metal-free hydrodefluorination: distinctive carbon-based reactivity. Chem Eur J. 2021;27(45):11730–11737. doi:10.1002/chem.202101534
Moghaddam KR, Aghapour G. One-pot, oxidative and selective conversion of benzylic silyl and tetrahydropyranyl ethers to gem-dichlorides using trichloroisocyanuric acid and triphenylphosphine as an efficient and neutral system. Phosphorus Sulfur Silicon Relat Elem. 2020;196(4):398–406. doi:10.1080/10426507.2020.1845680
DOI: https://doi.org/10.15826/chimtech.2022.9.2.02
Copyright (c) 2022 D.A. Shipilovskikh, M.F. Konkova, I.P. Nikonov, M.M Gladysheva, S.A. Shipilovskikh

This work is licensed under a Creative Commons Attribution 4.0 International License.
© Website Chimica Techno Acta, 2014–2024
ISSN 2411-1414 (Online)
This journal is licensed under a Creative Commons Attribution 4.0 International







