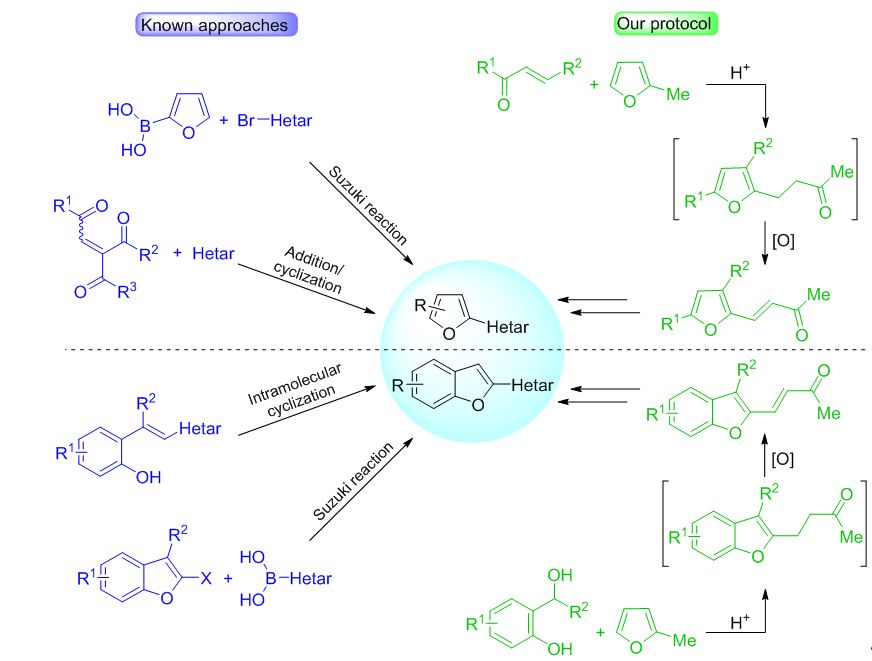
Synthetic strategy toward furyl- and benzofuryl-containing building blocks for organic materials
Abstract
Keywords
Full Text:
PDFReferences
Kumar B, Kaushik BK, Negi YS. Organic thin film transistors: structures, models, materials, fabrication, and applications. a review. Polym Rev. 2014;54(1):33–111. doi:10.1080/15583724.2013.848455
Tsang MP, Sonnemann GW, Bassani DM. Life-cycle assessment of cradle-to-grave opportunities and environmental impacts of organic photovoltaic solar panels compared to conventional technologies. Sol Energy Mater Sol Cells. 2016;156:37–48. doi:10.1016/j.solmat.2016.04.024
Cao W, Jiangeng X. Recent progress in organic photovoltaics: device architecture and optical design. Energy Environ Sci. 2014;7(7):2123–2144. doi:10.1039/c4ee00260a
Dong J, Chuxuan Y, Yingzhi C, Wenjie Z, Yushuan P, Yue Z, Lu-Ning W, Zhenghong H. Organic semiconductor nanostructures: optoelectronic properties, modification strategy, and photocatalytic applications. J Mater Sci Tech-nol. 2021;113:175–198. doi:10.1016/j.jmst.2021.09.002
Gonçalves JM, Martins PR, Rocha DP, Matias TA, Julião MS, Muñoz RA, Angnes L. Recent trends and perspectives in electrochemical sensors based on MOF-derived materials. J Mater Chem C. 2021;9:8718–8745. doi:10.1039/D1TC02025K
Yang D, Ma D. Development of organic semiconductor photodetectors: from mechanism to applications. Adv Opt Mater. 2018;7(1):1800522. doi:10.1002/adom.201800522
Marin ML, Santos-Juanes L, Arques A, Amat AM, Miranda MA. Organic photocatalysts for the oxidation of pollutants and model compounds. Chem Rev. 2011;112(3):1710–1750. doi:10.1021/cr2000543
Ates B, Koytepe S, Ulu A, Gurses C, Thakur VK. Chemistry, structures, and advanced applications of nanocomposites from biorenewable resources. Chem Rev. 2020;120(17):9304–9362. doi:10.1021/acs.chemrev.9b00553
Wang C, Dong H, Hu W, Liu Y, Zhu D. Semiconducting π-conjugated systems in field-effect transistors: a material odyssey of organic electronics. Chem Rev. 2011;112(4):2208–2267. doi:10.1021/cr100380z
Mei J, Diao Y, Appleton AL, Fang L, Bao Z. Integrated materials design of organic semiconductors for field-effect transistors. J Am Chem Soc. 2013;135(18):6724–6746. doi:10.1021/ja400881n
Zhang F, Xu X, Tang W, Zhang J, Zhuo Z, Wang J, Wang Y. Recent development of the inverted configuration organic solar cells. Sol Energy Mater Sol Cells. 2011;95(7):1785–1799. doi:10.1016/j.solmat.2011.02.002
Perepichka IF, Perepichka DF. Handbook of thiophene-based materials. New York: John Wiley & Sons;2009. 833 p. doi:10.1002/9780470745533
Zheng B, Huo L. Recent advances of furan and its derivatives based semiconductor materials for organic photovoltaics. Small Methods. 2021;5(9):2100493. doi:10.1002/smtd.202100493
Woo CH, Beaujuge PM, Holcombe TW, Lee OP, Fréchet JMJ. Incorporation of furan into low band-gap polymers for efficient solar cells. J Am Chem Soc. 2010;132(44):15547–15549. doi:10.1021/ja108115y
Song C, Sun D, Peng X, Bai J, Zhang R, Hou S, Xu Z. Dimeriza-tion of cyclopropenes to bifurans using tandem metal relay catalysis. Chem Commun. 2013;49(80):9167–9169. doi:10.1039/c3cc44762f
Nazim M, Ameen S, Akhtar MS, Seo HK, Shin HS. Furan-bridged thiazolo [5,4-d]thiazole based D–π–A–π–D type linear chromophore for solution-processed bulk-heterojunction organic solar cells. RSC Adv. 2015;5(9):6286–6293. doi:10.1039/c4ra13655a
Ramkumar V, Kannan P. Thiophene and furan containing pyrazoline luminescent materials for optoelectronics. J Lumin. 2016;169:204–215. doi:10.1016/j.jlumin.2015.09.020
Sun W, Wang C-H, Lv SF, Jiang JX, Guo X, Zhang F. Bis(benzofurano)pyrrole and hybrid thienopyrrole derivatives for organic thin-film transistors. Org Electron. 2019;77:105548. doi:10.1016/j.orgel.2019.105548
Chiummiento L, D’Orsi R, Funicello M, Lupattelli P. Last decade of unconventional methodologies for the synthesis of substituted benzofurans. Mol. 2020;25(10):2327. doi:10.3390/molecules25102327
Hussain M, Thai Hung N, Abbas N, Khera RA, Malik I, Pa-tonay T, Langer P. Synthesis of arylated benzofurans by regioselectivesuzuki-miyaura cross-coupling reactions of 2,3-dibromobenzofurans- and 2,3,5-tribromobenzofurans. J Het-erocycl Chem. 2014;52(2):497–505. doi:10.1002/jhet.2083
Cui K, Gao M, Zhao H, Zhang D, Yan H, Huang H. An efficient synthesis of aryl-substituted pyrroles by the suzuki–miyaura coupling reaction of SEM-protected pyrroles. Mol. 2019;24(8):1594. doi:10.3390/molecules24081594
Brucoli F, Guzman JD, Maitra A, James CH, Fox KR, Bhakta S. Synthesis, anti-mycobacterial activity and dna sequence-selectivity of a library of biaryl-motifs containing polyamides. Bioorg Med Chem. 2015;23(13):3705–3711. doi:10.1016/j.bmc.2015.04.001
Yang Y, Gao M, Wu LM, Deng C, Zhang DX, Gao Y, Wu AX. A facile synthesis of indole–furan conjugates via integration of convergent and linear domino reactions. Tetrahedron. 2011;67(29):5142–5149. doi:10.1016/j.tet.2011.05.058
Thwin M, Mahmoudi B, Ivaschuk OA, Yousif QA. An efficient and recyclable nanocatalyst for the green and rapid synthesis of biologically active polysubstituted pyrroles and 1,2,4,5-tetrasubstituted imidazole derivatives. RSC Adv. 2019;9(28):15966–15975. doi:10.1039/c9ra02325a
Jana A, Das Adhikary N, Pramanik A. Graphene oxide (GO) catalysed MW-assisted one-pot synthesis of densely substituted furan. Green Chem. 2020;22: 4304–4310. doi:10.1039/d0gc00723d
Serrano-Ruiz JC, Luque R, Sepúlveda-Escribano A. Trans-formations of biomass-derived platform molecules: from high added-value chemicals to fuels via aqueous-phase processing. Chem Soc Rev. 2011;40(11):5266–5281. doi:10.1039/c1cs15131b
Abaev VT, Trushkov IV, Uchuskin MG. The butin reaction. Chem Heterocycl Compd. 2016;52(12):973–995. doi:10.1007/s10593-017-1996-x
Fadeev AA, Makarov AS, Uchuskin MG. Acid-catalyzed cascade reaction of 2-alkylfurans with α,β-unsaturated ketones: a shortcut to 2,3,5-trisubstituted furans. J Org Chem. 2021;86(23):17362–17370. doi:10.1021/acs.joc.1c01692
Makarov AS, Kekhvaeva AE, Chalikidi PN, Abaev VT, Trushkov IV, Uchuskin MG. A simple synthesis of densely substituted benzofurans by domino reaction of 2-hydroxybenzyl alcohols with 2-substituted furans. Synthesis. 2019;51(19):3747–3757. doi:10.1055/s-0039-1690000
Albuquerque HMT, Santos CMM, Cavaleiro JAS, Silva AMS. Chalcones as versatile synthons for the synthesis of 5- and 6-membered nitrogen heterocycles. Curr Org Chem. 2014;18(21):2750–2775. doi:10.2174/1385272819666141013224253
Bagdi A, Pattanayak P, Paul S, Mitra M, Choudhuri T, Sheikh AS. Application of conjugated carbonyls in the synthesis of heterocycles via oxidative cycloaddition and cyclization reactions. Adv Synth Catal. 2020;362(24):5601–5621. doi:10.1002/adsc.202000970
Kuznetsov AN, Makarov AS, Rubtsov AE, Butin AV, Gevorgyan V. Brönsted acid-catalyzed one-pot synthesis of indoles from O-aminobenzyl alcohols and furans. J Org Chem. 2013;78(23):12144–12153. doi:10.1021/jo402132p
Merkushev AA, Strel’nikov VN, Uchuskin MG, Trushkov IV. A simple synthesis of benzofurans by acid-catalyzed domino reaction of salicyl alcohols with N-tosylfurfurylamine. Tetrahedron. 2017;73(46):6523–6529. doi:10.1016/j.tet.2017.09.043
Chen H, Liu L, Huang T, Chen J, Chen T. Direct dehydrogenation for the synthesis of α,β‐unsaturated carbonyl compounds. Adv Synth Catal. 2020;362(16):3332–3346. doi:10.1002/adsc.202000454
Alsharif MA, Raja QA, Majeed NA, Jassas RS, Alsimaree AA, Sadiq A, Naeem N, Mughal EU, Alsantali RI, Moussa Z, Ahmed SA. DDQ as a versatile and easily recyclable oxidant: a systematic review. RSC Adv. 2021;11:29826. doi:10.1039/d1ra04575j
Walker D, Hiebert JD. 2,3-dichloro-5,6-dicyanobenzoquinone and its reactions. Chem Rev. 1967;67(2):153–195. doi:10.1021/cr60246a002
Mathiyazhagan AD, Anilkumar G. Recent advances and ap-plications of p-toluenesulfonylmethyl isocyanide (TosMIC). Org Biomol Chem. 2019;17:6735–6747. doi:10.1039/c9ob00847k
Ma Z, Ma Z, Zhang D. Synthesis of multi-substituted pyrrole derivatives through [3+2] cycloaddition with tosylmethyl isocyanides (TosMICs) and electron-deficient compounds. Mol. 2018;23(10):2666. doi:10.3390/molecules23102666
Kaur T, Wadhwa P, Sharma A. Arylsulfonylmethyl isocyanides: a novel paradigm in organic synthesis. RSC Adv. 2015;5(65):52769–52787. doi:10.1039/c5ra07876h
Kumar K. TosMIC: A powerful synthon for cyclization and sulfonylation. Chem Sel. 2020;5(33):10298–10328. doi:10.1002/slct.202001344
DOI: https://doi.org/10.15826/chimtech.2022.9.4.03
Copyright (c) 2022 Diana A. Eshmemet’eva, Danil K. Vshivkov, Yury A. Vasev, Danil A. Myasnikov

This work is licensed under a Creative Commons Attribution 4.0 International License.
Chimica Techno Acta, 2014–2025
eISSN 2411-1414
Copyright Notice







