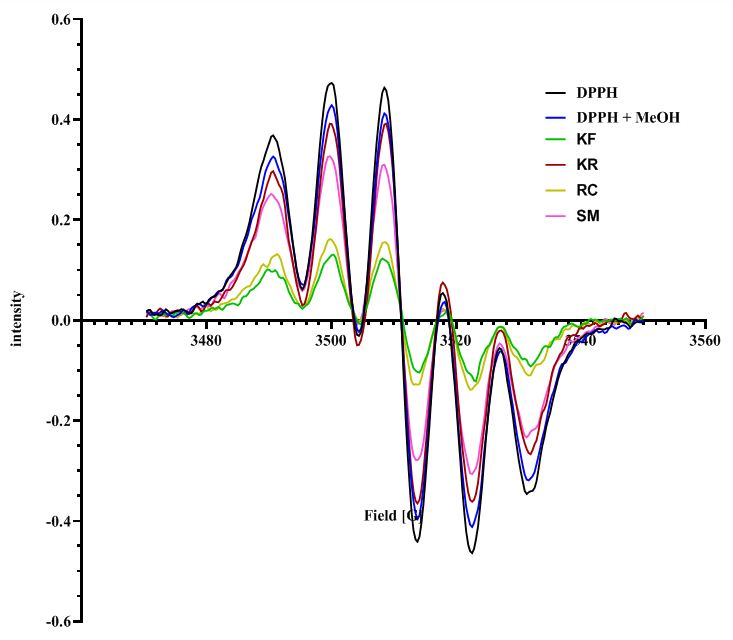
Phytochemical screening and properties of botanical crude extracts and ethyl acetate fractions isolated by deep eutectic solvent
Abstract
Keywords
Full Text:
PDFReferences
Yang L, Chen J, Lu H, Lai J, He Y, Liu S, Guo X. Pueraria lobata for Diabetes Mellitus: Past, Present and Future. Am J Chin Med. 2019;47(7):1419–1444. doi:10.1142/S0192415X19500733
Boyle C, Seac U, Moizer K, Barlow T, Jeffery B. Phytoestrogens and health. Woodhead Publishing. Limited;2003.
Szeja W, Grynkiewicz G, Rusin AJCOC. Isoflavones, their glycosides and glycoconjugates. Synthesis and biological activity. 2017;21(3):218–235. doi:10.2174/1385272820666160928120822
B. Hohman, US Department of Agriculture Natural Resources Conservation Service. https://www.nrcs.usda.gov/wps/portal/nrcs/site/national/home/.
Son E, Yoon JM, An BJ, Lee YM, Cha J, Chi GY, Kim DS. Comparison among Activities and Isoflavonoids from Pueraria thunbergiana Aerial Parts and Root. Mol. 2019;24(5):912. doi:10.3390/molecules24050912
Dénes A, Papp N, Babai D, Czúcz B, Molnár ZJASBP. Wild plants used for food by Hungarian ethnic groups living in the Carpathian Basin. Acta Societatis Botanicorum Poloniae. 2012;81(4):381–396. doi:10.5586/ASBP.2012.040
Svanberg I. The use of wild plants as food in preindustrial Sweden. Acta Societatis Botanicorum Poloniae. 201;81(4):317–327. doi:10.5586/ASBP.2012.039
Atkinson C, Compston, Day NE, Dowsett M, Bingham JE. The effects of phytoestrogen isoflavones on bone density in women: a double-blind, randomized, placebo-controlled trial. Am J Clin Nutrit. 2004;79(2):326–333. doi:10.1093/AJCN/79.2.326
Miadoková E. Isoflavonoids—an overview of their biological activities and potential health benefits. Interdisciplin Toxicol. 2009;2(4):211–218 doi:10.2478/v10102-009-0021-3
Krenn L, Unterrieder I, Renate Ruprechter. Quantification of isoflavones in red clover by high-performance liquid chromatography. 2002;777(1–2):123–128. doi:10.1016/S1570-0232(02)00079-X
Kaurinovic B, Popovic M, Vlaisavljevic S, Schwartsova H, Vojinovic-Miloradov M. Antioxidant profile of Trifolium pratense L. Mol. 2012;17(9):11156–11172. doi:10.3390/molecules170911156
Adlercreutz H, Mazur W. Phyto-oestrogens and Western diseases. Ann Med. 1997;29(2):95–120. doi:10.3109/07853899709113696
Batista VSF, Nunes GL, Viegas GI, Lucas BN, Bochi VC, Emanuelli T, Barin JS, de Menezes CR, da Rosa CS. Extraction, characterization and microencapsulation of isoflavones from soybean molasses. Ciencia Rural. 2020;50(3). doi:10.1590/0103-8478cr20190341
Kwun KH, Kim GJ, Shin HJ. Ultrasonication assistance increases the efficiency of isoflavones extraction from kudzu (Pueraria lobata Ohwi) roots waste. Biotechnol Bioprocess Eng. 2009;14(3):345–348. doi:10.1007/s12257-008-0199-9
Prasain JK, Jones K, Kirk M, Wilson L, Smith-Johnson M, Weaver C, Barnes S. Profiling and quantification of isoflavonoids in kudzu dietary supplements by high-performance liquid chromatography and electrospray ionization tandem mass spectrometry. J Agricult Food Chem. 2009;51(15):4213–4218. doi:10.1021/jf030174a
Blicharski T, Oniszczuk A. Extraction methods for the isolation of isoflavonoids from plant material. Open Chem. 2017;15(1):34–45. doi:10.1515/chem-2017-0005
Butt Y, Kurdowska A, Allen TC. Acute lung injury: A clinical and molecular review. Arch Pathol Lab Med. 2016;140(4):345–350. doi:10.5858/arpa.2015-0519-RA
Zhao B-Y, Xu P, Yang F-X, Wu H, Zong M-H, Lou W-Y. Bio-compatible deep eutectic solvents based on choline chloride: characterization and application to the extraction of rutin from Sophora japonica. ACS Sustain Chem Eng. 2015;3(11):2746–2755. doi:10.1021/acssuschemeng.5b00619
Duru KC, Slesarev GP, Aboushanab SA, Kovalev IS, Zeidler DM, Kovaleva EG, Bhat R. An ecofriendly approach to enhance the extraction and recovery efficiency of isoflavones from kudzu roots and soy molasses wastes using ultra-sound-assisted extraction with natural deep eutectic solvents (NADES). Ind Crops Prod. 2022;182:114886. doi:10.1016/j.indcrop.2022.114886
Shang X, Dou Y, Zhang Y, Tan JN, Liu X, Zhang Z. Tailor-made natural deep eutectic solvents for green extraction of isoflavones from chickpea (Cicer arietinum L.) sprouts. Ind Crops Prod. 2019;140. doi:10.1016/j.indcrop.2019.111724
Chen TR, Shih SC, Ping HP, Wei QK. Antioxidant activity and isoflavonoid components in different sections of Pueraria lobata root. J Food Drug Anal. 2012;20(3):681–685. doi:10.6227/jfda.2012200316
Tian F, Li B, Ji B, Yang J, Zhang G, Chen Y, Luo Y. Antioxidant and antimicrobial activities of consecutive extracts from Galla chinensis: The polarity affects the bioactivities. Food Chem. 2009;113(1):173–179. doi:10.1016/J.FOODCHEM.2008.07.062
Feresin RG, Pourafshar S, Huang J, Zhao Y, Arjmandi BH, Salazar G. Extraction and purification of polyphenols from freeze-dried berry powder for the treatment of vascular smooth muscle cells in vitro. J Visual Exp. 2017;125:55605. doi:10.3791/55605
Cicco N, Lanorte MT, Paraggio M, Viggiano M, Lattanzio V. A reproducible, rapid and inexpensive Folin-Ciocalteu micro-method in determining phenolics of plant methanol extracts. Microchem J. 2009;91(1):107–110. doi:10.1016/j.microc.2008.08.011
Zhang Y, Chen J, Zhang C, Wu W, Liang X. Analysis of the estrogenic components in kudzu root by bioassay and high performance liquid chromatography. J Steroid Biochem Mol Biol. 2005;94(4):375–381. doi:10.1016/j.jsbmb.2004.10.022
Prajapati R, Park SE, Park HJ, Jung HA, Choi JS. Identification of a Potent and Selective Human Monoamine Oxidase-A Inhibitor, Glycitein, an Isoflavone Isolated from Pueraria lobata Flowers. ACS Food Sci Technol. 2021;1(4):538–550. doi:10.1021/acsfoodscitech.0c00152
Gu L, Gu W. Characterisation odsoy isoflavones and screening for novel malonyl glycosides using high-performance liquid chromatography-electrospray ionization mass spectrometry. Phytochem Anal. 2001;12(6):377–382. doi:10.1002/pca.603
Boué SM, Wiese TE, Nehls S, Burow ME, Elliott S, Carter-Wientjes CH, Shih BY, McLachlan JA, Cleveland TE. Evaluation of the estrogenic effects of legume extracts containing phytoestrogens. J Agricult Food Chem. 2003;51(8):2193–2199. doi:10.1021/jf021114s
Filipiak-Szok A, Kurzawa M, Szłyk E. Determination of antioxidant capacity and content of phenols, phenolic acids, and flavonols in Indian and European gooseberry. Chem Papers. 2012;66(4):259–268. doi:10.2478/s11696-012-0151-5
Kayano S-I, Matsumura Y, Kitagawa Y, Kobayashi M, Nagayama A, Kawabata N, Kikuzaki H, Kitada Y. Isoflavone C-glycosides isolated from the root of kudzu (Pueraria lobata) and their estrogenic activities. Food Chem. 2012;134(1):282–287. doi:10.1016/j.foodchem.2012.02.137
Saviranta NM, Anttonen MJ, von Wright A, Karjalainen R. Agriculture, Red clover (Trifolium pratense L.) isoflavones: determination of concentrations by plant stage, flower colour, plant part and cultivar. J Sci Food Agricult. 2008;88(1):125–132. doi:10.1002/JSFA.3056
Tamer U, Adigüzel N, Küçükboyaci N, Torul H, Bani B. Determination of Isoflavone Content by HPLC UV Method and In Vitro Antioxidant Activity of Red Clover Trifolium Pratense L. 2014.
Burdette CQ, Marcus RK. Determination of isoflavone content in soy, red clover, and kudzu dietary supplement materials by liquid chromatography-particle beam/electron ionization mass spectrometry. J AOAC Int. 2013;96(5):925–932. doi:10.5740/jaoacint.12-431
Procházková D, Boušová I, Wilhelmová N. Antioxidant and prooxidant properties of flavonoids. Fitoterap. 2011;82(4):513–523. doi:10.1016/j.fitote.2011.01.018
Bourgou S, Ksouri R, Bellila A, Skandrani I, Falleh H, Marzouk B. Phenolic composition and biological activities of Tunisian Nigella sativa L. shoots and roots. Comptes Rendus Biol. 2008;331(1):48–55. doi:10.1016/j.crvi.2007.11.001
DOI: https://doi.org/10.15826/chimtech.2022.9.4.04
Copyright (c) 2022 Saied Aboushanab, Vadim Shevyrin, Mustapha Kamel, Jonas Kambele, Elena Kovaleva

This work is licensed under a Creative Commons Attribution 4.0 International License.
Chimica Techno Acta, 2014–2025
eISSN 2411-1414
Copyright Notice







