Catalytic activity and selectivity of Palladium and Nickel catalysts in hydrogenation reactions of nitro- and acetylene compounds
Abstract
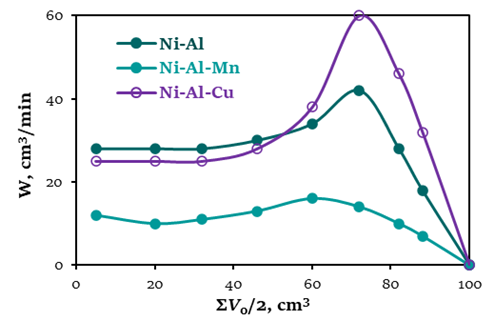
This paper presents the results of a study on the catalytic activity and selectivity of nickel and palladium catalysts in hydrogenation reactions of nitro- and acetylene compounds. It was shown that the activity and selectivity of nickel catalysts in the hydrogenation of phenylacetylene depend on the nature of modifying additives (Cu, Zn, Ti, Cr, Bi, Ti–Cu, Mn, Fe), and the activity and selectivity of palladium catalysts based on a polymer of metal complexes depends on the method of their preparation. It was found that for certain concentrations of the active phase of palladium (0.8 wt.%) and the polymer of potassium humate (1.0 wt.%.) in the catalyst, where palladium and the polymer were deposited on bauxite-094 together, the catalyst exhibits the greatest activity and selectivity when hydrogenating phenylacetylene and potassium orthonitrophenolate.
Keywords
Full Text:
PDFReferences
Sagdeev KA, Sagdeev AA. Hydrogenation of unsaturated hydrocarbons on a palladium catalyst. Bull Technol Univer [Internet]. 2015;18:12:32–34. Russian. Available from: https://www.elibrary.ru/item.asp?id=23829287
Vreschagina NV, Zakharova GB, Antonova TN, Abramov IG. Liquid-phase hydrogenation of cycloolefins. Chem Chem Technol [Internet]. 2013; 56:12:79–82. Russian. Available from: https://www.elbrary.ru/item.asp?id=21008020
Nikolaev SA, Zaveskin LN, Smirnov VV, Averyanov VA, Zaveskin KL. Catalytic hydrogenation of alkyne and alkadiene impurities from alkenes. Practical theoretical aspects. Russ Chem Rev. 2009;78(3):231–247. doi:10.1070/RC2009v078n03ABEH003893
Spiridinov VS, Vasilkov AYu, Podshibikhin VL, Serdan AA, Naumkin AV, Lisichkin GV. Metal-vapour synthesis of grafted mono- and bimetallic nanoclusters of gold, nickel and palladium and their hexane-1 hydration and chlorbenzene hydrodechlorination catalytic activity. Chem Chem Technol [Internet]. 2007;50(8):108–111. Russian. Available from: https://www.elibrary.ru/item.asp?id=9532418
Stytsenko VD, Melnikov DP. Selective hydrogenation of diene and acetylene compounds on metal-containing catalysts. J Phys Chem. 2016;90(5):691–702. doi:10.7868/S0044453716040300
Anjan Kumar GC, Bodke YD, Manjunatha B, Satyanarayan ND, Nippu BN, Muthipeedika Nibin Joy. Novel synthesis of 3-(Phenyl) (ethylamino) methyl)-4-hydroxy-2H-chromen-2-one derivatives using biogenic ZnO nanoparticles and their applications. Chim Techno Acta. 2022;9(1):20229104. doi:10.15826/chimtech.2022.9.1.04
Ulomsky EN, Lyapustin DN, Fedotov VV, El’tsov OS, Sapozhnikova IM, Kozhevnikov DN, Mukhin EM. The features of nucleophilic substitution of the nitro group in 4-alkyl-6-nitro-1,2,4-triazolo[5,1-c][1,2,4]triazines. Chim Techno Acta. 2017;4(1):11–24. doi:10.15826/chimtech.2017.4.1.020
Magdalinova NA, Klyuev MV. Hydrogenation of nitro- and unsaturated organic compounds on catalysts containing nanoscale palladium particles. Petrochem. 2017;57(6):647–652. doi:10.7868/S0028242117060065
Nikolaev SA, Permyakov NA, Smirnov VV, Vasil’kov AYu, Lanin SN. Selective hydrogenation of phenylacetylene into styrene on gold nanoparticles. Kinet Catal. 2010;51(2)288–292. doi:10.1134/S0023158410020187
Zharmagambetova AK, Seitkaliev KS, Talgatov ET, Auezkhanov AS, Jardimalieva GI, Pomogailo AD. Polymer-modified supported palladium catalysts for the hydrogenation of acetylene compounds. Kinet Catal. 2016;57:3:360–367. doi:10.1134/S0023158416030174
Pomogailo AD. Polimernye immobilizovannye metallokompleksnye katalizatory [Polymer immobilized metal–complex catalysts]. Moscow: Nauka; 1988. 303 p. Russian
Yermoldina E, Kairbekov Zh, Jeldybayeva I, Vassilina G, Sabitova A. A Study of catalytic activity of palladium catalysts with potassium humate in hydrogenation reactions. In: International Conference on Artificial Intelligence and Bioengineering (ICAIB2016); 2016 July 24–25; Bangkok, Thailand. P. 3657. doi:10.12783/DTCSE/CMSAM2016/3657
Suimbayeva S, Kairbekov Zh, Jeldybayeva I, Ermoldina E. Liquid-phase hydrogenation of 1-hexene and phenylacetylene over multicomponent nickel skeleton catalysts. In: MATEC Web of Conferences; 2021;340:01010. doi:10.1051/matecconf/202134001010
Taguchi A, Schuth F. Ordered mesoporous materials in Catalysis. Micropor Mesopor Mater. 2005;77(1)1–45. doi:10.1016/j.micromeso.2004.06.030
Perego C, Millini R. Porous materials in catalysis: challenges for mesoporous materials. Chem Soc Rev. 2013;42:3956–3976. doi:10.1039/C2CS35244C
Karakhanov E, Kardasheva Yu, Kulikov L, Maximov A, Zolotukhina A, Vinnikova M, Ivanov A. Sulfide on porous aromatic frameworks for naphthalene hydroprocessing. Catal. 2016;6(8):122. doi:10.3390/catal6080122
Lysenko SV, Kryukov IO, Sarkisov OA, Abikenova AB, Baranova SV, Ostroumova VA, Kardashev SV, Kulikov AB, Karakhanov EA. Mesoporous aluminosilicates as components of gas oil cracking and higher-alkane hydroisomerization catalysts. Petroleum Chem. 2011;51(3):151–156. doi:10.1134/S0965544111030091
DOI: https://doi.org/10.15826/chimtech.2022.9.3.06
Copyright (c) 2022 Indira M. Jeldybayeva, Zhaksyntay K. Kairbekov, Kazhmukan O. Kishibayev, Elmira T. Yermoldina, Saltanat M. Suimbayeva

This work is licensed under a Creative Commons Attribution 4.0 International License.
Chimica Techno Acta, 2014–2025
eISSN 2411-1414
Copyright Notice







