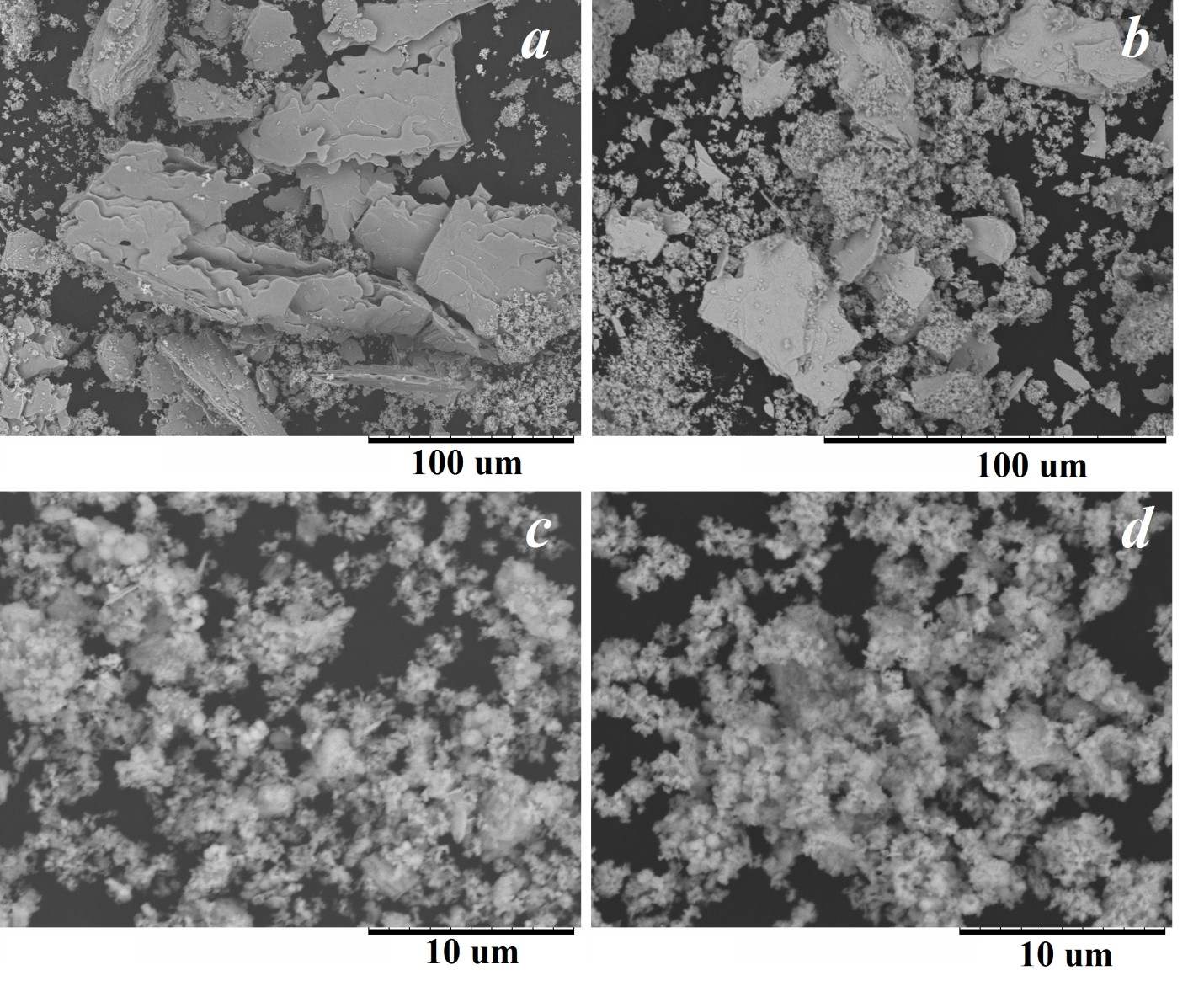
Thermal transformations of bismuth (III) tartrates
Abstract
Keywords
Full Text:
PDFReferences
Ubale MB, Betallu MA, Tadke VB, Vhankate SM, Pathade GR. Synthesis, characterisation and in vitro antimicrobial activity of mixed “transition metal–Barium Tartarate” complexes. World J Pharm Res. 2016;5(6):1578–1594. doi:10.20959/wjpr20166-6361
Betallu MA, Tadke VB, Pathade GR, Sapnar KB, Ubale MB. Synthesis, characterisation and microbial activity of mixed “transition metal-Calcium tartarate” complexes. J Appl Chem. 2016;5(1):165–178.
Mathivanan V, Haris M, Chandrasekaran J. Thermal, magnetic, dielectric and anti-microbial properties of solution-grown pure and doped sodium potassium tartrate crystals. Optik. 2016;127(4):1804–1808. doi:10.1016/j.ijleo.2015.11.092
Jian X, Cao Y, Chen G, Wang C, Tang H, Yin L, Zhang C. High-purity Cu nanocrystal synthesis by a dynamic decomposition method. Nanoscale Res Lett. 2014;9(1):1–9. doi:10.1186/1556-276X-9-689
Li T, Liu Y, Ma G, He D. Spherical and radiate Ni particles prepared by the tartrate precipitation and thermal decomposition method. J Wuhan Univ Technol Mater Sci Ed. 2013;28(5):857–861. doi:10.1007/s11595-013-0782-6
Palacios-Hernández T, Hirata-Flores GA, Contreras-López OE, Mendoza-Sánchez ME, Valeriano-Arreola I, González-Vergara E, Méndez-Rojas MA. Synthesis of Cu and Co metal oxide nanoparticles from thermal decomposition of tartrate complexes. Inorg Chim Acta. 2012;392:277–282. doi:10.1016/j.ica.2012.03.039
Bhattacharjee CR, Purkayastha DD, Das N. Surfactant-free thermal decomposition route to phase pure tricobalt tetraoxide nanoparticles from cobalt (II)-tartrate complex. J Sol-Gel Sci Technol. 2013;65(3):296–300. doi:10.1007/s10971-012-2935-z
Reddy JR, Ravi G, Suresh P, Veldurthi NK, Velchuri R, Vithal M. Antimony potassium tartrate. J Therm Anal Calorim. 2014;115(2):1321–1327. doi:10.1007/s10973-013-3502-8
Yang JM, Yang KL. An optimal low-temperature tartrate precursor method for the synthesis of monophasic nanosized ZnFe2O4. J Nanopart Res. 2009;11(7):1739–1750. doi:10.1007/s11051-008-9537-2
Wang R, Li H, Ip TKY, Sun H. Bismuth drugs as antimicrobial agents. Adv Inorg Chem. 2020;75:183–205. doi:10.1016/bs.adioch.2019.10.011
Gu L, Li S, He Y, Chen Y, Jiang Y, Peng Y, Yang H. Bismuth, rabeprazole, amoxicillin, and doxycycline as first-line Helicobacter pylori therapy in clinical practice: A pilot study. Helicobacter. 2019;24(4):e12594. doi:10.1111/hel.12594
Loh A, Ong YC, Blair VL, Kedzierski L, Andrews PC. Bismuth (III) α-hydroxy carboxylates: highly selective toxicity of glycolates towards Leishmania major. J Biol Inorg Chem. 2015;20(7):1193–1203. doi:10.1007/s00775-015-1299-6
Tolokonnikova LI, Ainekenova RR, inventors; Institute of Inorganic and Physical Chemistry, Academy of Sciences of the Kyrgyz SSR, assignee. Sposob polucheniya oksida vismuta. SU certificate of authorship 1608124 А1. 1990 Nov 23. Russian.
Ahadiat G, Tabatabaee M, Gholivand K, Zare K, Dusek M, Kucerakova M. A two-dimensional bismuth coordination polymer with tartaric acid: Synthesis, characterization and thermal decomposition to Bi2O3 nanoparticles. Main Group Chem. 2017;16(1):7–16. doi:10.3233/MGC-160216
Bedoya Hincapie CM, Pinzon Cardenas MJ, Alfonso Orjuela JE, Restrepo Parra E, Olaya Florez JJ. Physical-chemical properties of bismuth and bismuth oxides: Synthesis, characterization and applications. Dyna. 2012;79(176):139–148.
Camilleri J, Borg J, Damidot D, Salvadori E, Pilecki P, Zaslansky P, Darvell BW. Colour and chemical stability of bismuth oxide in dental materials with solutions used in routine clinical practice. PLoS One. 2020;15(11):e0240634. doi:10.1371/journal.pone.0240634
Selvamani T, Anandan S, Granone L, Bahnemann DW, Ashokkumar M. Phase-controlled synthesis of bismuth oxide polymorphs for photocatalytic applications. Mater Chem Front. 2018;2(9):1664–1673. doi:10.1039/C8QM00221E
Gramm G, Fuhrmann G, Wieser M, Schottenberger H, Huppertz H. Environmentally benign inorganic red pigments based on tetragonal β-Bi2O3. Dyes Pigments. 2019;160:9–15. doi:10.1016/j.dyepig.2018.07.039
Zou J, Yu Z. Yellow β-Bi2O3/BaCO3 complex pigments with impressive near infrared reflectance and excellent color performance. Sol Energy Mater Sol Cells. 2019;199:99–107. doi:10.1016/j.solmat.2019.04.031
Yukhin YuM, Mikhaylov YuI. Khimiya vismutovykh soyedineniy i materialov. Moscow: Publishing House of the Siberian Branch of the Russian Academy of Sciences; 2001. 360 p. Russian.
Timakova EV, Logutenko OA, Evseenko VI, Mikhailova AS, Yukhin YuM. Izucheniye sostava substantsii antibakterial'nogo preparata Bismoverol, poluchennogo osazhdeniyem tartratov vismuta (III) iz rastvorov kislot. Khimiya v interesakh ustoychivogo razvitiya. 2015;23(4):379–387. Russian. doi:10.15372/KhUR20150407
Hasmuddin M, Abdullah M.M, Singh P, Shkir M, Vijayan N, Wahab M.A. Ab-initio study of L-Tartaric Acid (LTA) single crystal for NLO application. Opt Laser Technol. 2015;74:53–59. doi:10.1016/j.optlastec.2015.05.013
Khan N, Vijayan N, Shandilya K, Kumar R, Krishna A, Chopra S, Yadav S, Moona G, Jewariya M. Single crystal growth of L-tartaric acid and its characterization for optical applications. J Mater Sci Mater Electron. 2020;31(6):4494–4502. doi:10.1007/s10854-020-02998-4
Tumkin II, Khairullina EM, Myund LA, Logunov LS, Gordeychuk DI, Panov MS, Kochemirovsky VA. Spectroscopic and theoretical studies of potassium sodium L(+)-tartrate tetrahydrate and L-tartaric acid used as precursors for in situ laser-induced deposition of the catalytically active copper microstructures. Opt Quantum Electron. 2019;51(3):1–11. doi:10.1007/s11082-019-1800-5
Chandran S, James GJ, Magesh M, Prasanna N. Synthesis, crystal growth, structural, spectral, laser threshold energy and dielectric properties of lithium L-tartrate monohydrate crystal. J Mol Struct. 2021;1223:128988. doi:10.1016/j.molstruc.2020.128988
Mathivanan V, Haris M. Studies on solution-grown pure and doped sodium potassium tartrate crystals. Spectrochim Acta, Part A. 2013;102:341–349. doi:10.1016/j.saa.2012.10.033
Xiao J, Zhang H, Xia Y, Li Z, Huang W. Rapid and high-capacity adsorption of sulfonated anionic dyes onto basic bismuth (III) nitrate via bidentate bridging and electrostatic attracting interactions. RSC Adv. 2016;6(46):39861–39869. doi:10.1107/ 10.1039/C6RA03055F
Krishnakumar V, Dheivamalar S. Growth and vibrational spectroscopic studies of strontium tartrate (C4H4O6Sr): a nonlinear optical single crystal. J Raman Spectrosc. 2009;40(6):627–631. doi:10.1002/jrs.2173
Ventruti G, Scordari F, Bellatreccia F, Della Ventura G, Sodo A. Calcium tartrate esahydrate, CaC4H4O6·6H2O: a structural and spectroscopic study. Acta Crystallogr Sec B Struct Sci. 2015;71(1):68–73. doi:10.1107/S2052520614027516
Asanov IP, Asanova TI, Bulusheva LG, Shlyakhova EV, Okotrub AV, Flahaut E. Thermal decomposition of Co-doped calcium tartrate and use of the products for catalytic chemical vapor deposition synthesis of carbon nanotubes. J Phys Chem C. 2012;116(1):343–351. doi:10.1021/jp2092169
Patil HM, Sawant DK, Bhavsar DS, Patil JH, Girase KD. FTIR and thermal studies on gel grown neodymium tartrate crystals. J Therm Anal Calorim. 2012;107(3):1031–1037. doi:10.1007/s10973-011-1599-1
Timakova EV, Afonina LI, Bulina NV, Shatskaya SS, Yukhin YuM, Volodin VA. Synthesis of basic bismuth (III) oxalate by precipitation from nitrate solutions. Russ J Appl Chem. 2017;90(7):1040–1046. doi:10.1134/S1070427217070035
DOI: https://doi.org/10.15826/chimtech.2022.9.3.15
Copyright (c) 2022 Liubov I. Afonina, Tatiana E. Timakova, Evgeniya V. Timakova, Konstantin B. Gerasimov, Yuri M. Yukhin

This work is licensed under a Creative Commons Attribution 4.0 International License.
Chimica Techno Acta, 2014–2025
eISSN 2411-1414
Copyright Notice







