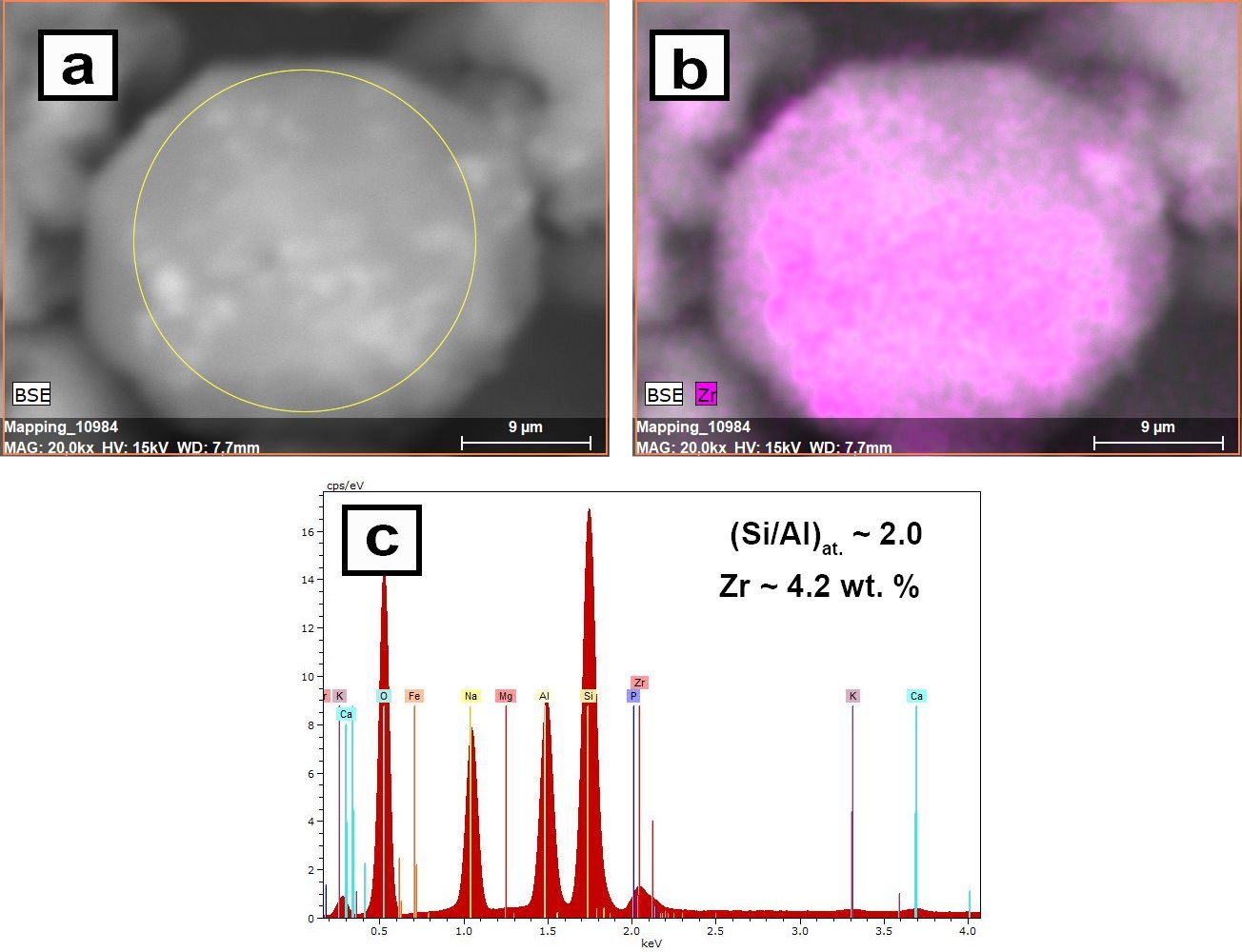
Hydrothermal synthesis and sorption performance to Cs(I) and Sr(II) of zirconia-analcime composites derived from coal fly ash cenospheres
Abstract
Keywords
Full Text:
PDFReferences
International Atomic Energy Agency, Application of Ion Exchange Processes for the Treatment of Radioactive Waste and Management of Spent Ion Exchangers. Tech. Rep. Ser. No. 408. Vienna: IAEA; 2002. 124 p.
Clearfield A. Inorganic ion exchangers, past, present, and future. Solvent Extr Ion Exch. 2000;18:655–678. doi:10.1080/07366290008934702
El-Kamash AM. Evaluation of zeolite A for the sorptive removal of Cs+ and Sr2+ ions from aqueous solutions using batch and fixed bed column operations. J Hazard Mater. 2008;151:432–445. doi:10.1016/j.jhazmat.2007.06.009
Figueiredo BR, Cardoso SP, Portugal I, Rocha J, Silva CM, Inorganic ion exchangers for cesium removal from radioactive wastewater. Separ Purif Rev. 2018;47:306–336. doi:10.1080/15422119.2017.1392974
Peters TB, Barnes MJ, Hobbs DT, Walker DD, Fondeur FF, Norato MA, Fink SD, Pulmano RL. Strontium and actinide separations from high level nuclear waste solutions using monosodium titanate 2. Actual Waste Testing. Separ Sci Technol. 2006;41:2409–2427. doi:10.1080/01496390600742963
Pichot E, Dacheux N, Brandel V, Genet M. Investigation of 137Cs+, 85Sr2+ and 241Am3+ ion exchange on thorium phosphate hydrogenphosphate and their immobilization in the thorium phosphate diphosphate. New J Chem. 2000;24:1017–1023. doi:10.1039/B006022O
Saeb S, Patchet SJ. Radioactive Waste Disposal (Geology). Editor(s): Robert A. Meyers, Encyclopedia of Physical Science and Technology (3nd ed.). Moscow: Academic Press; 2003. P. 633–641. doi:10.1016/B0-12-227410-5/00641-4
Vereshchagina TA, Fomenko EV, Vasilieva NG, Solovyov LA, Vereshchagin SN, Bazarova ZG, Anshits AG. A novel layered zirconium molybdate as a precursor to a ceramic zircono-molybdate host for lanthanide bearing radioactive waste. J. Mater. Chem. 2011;21:12001–12007. doi:10.1039/C1JM11202C
Mimura H, Akiba K, Ozawa M. Preparation of ceramic solid forms immobilizing cesium and/or strontium and evaluation of their physical and chemical properties. In: Proc Inter Conf Nuclear Energy for New Europe; 2002 Sep 9–12; Kranjska Gora, Slovenia. p. 62640.
Dosch RG. Ceramic from ion exchangers: an approach to nuclear waste solidification. Trans Amer Nucl. Soc. 1975;22:355–357.
Guo B, Kamura Y, Koilraj P, Sasaki K. Co-sorption of Sr2+ and SeO42− as the surrogate of radionuclide by alginate-encapsulated graphene oxide-layered double hydroxide beads. Environ. Res. 2020;187:109712. doi:10.1016/j.envres.2020.109712
Attallah MF, Hassan HS, Youssef MA. Synthesis and sorption potential study of Al2O3–ZrO2–CeO2 composite material for removal of some radionuclides from radioactive waste effluent. Appl Radiat Isot. 2019;147:40–47.doi:10.1515/ract-2019-3221
Youssef MA, El-Naggar MR, Ahmed IM, Attallah MF. Batch kinetics of 134Cs and 152+154Eu radionuclides onto poly-condensed feldspar and perlite based sorbents. J. Hazard Mater. 2021;403:123945. doi:10.1016/j.jhazmat.2020.123945
Voronina AV, Noskova AYu, Semenishchev VS, Gupta DK. Decontamination of seawater from 137Cs and 90Sr radionuclides using inorganic sorbents. J Environ Radioact. 2020;217:106210. doi:10.1016/j.jenvrad.2020.106210
Mahmoud MR, Seliman AF. Evaluation of silica/ferrocyanide composite as a dual-function material for simultaneous removal of 137Cs+ and 99TcO4− from aqueous solutions. Appl Radiat Isot. 2014;91:141–154. doi:10.1016/j.apradiso.2014.05.021
Nayl AA, Ahmed IM, Abd-Elhamid AI, Aly HF, Attallah MF. Selective sorption of 134Cs and 60Co radioisotopes using synthetic nanocopper ferrocyanide-SiO2 materials. Sep Purif Technol. 2020;234:116060. doi:10.1016/j.seppur.2019.116060
Attallah MF, Youssef MA, Imam DM. Preparation of novel nano composite materials from biomass waste and their sorptive characteristics for certain radionuclides. Radiochim Acta. 2020;108:137149. doi:10.1515/ract-2019-3108
Breck DW. Zeolite Molecular Sieves: Structure, Chemistry, and Use. New York: John Wiley & Sons; 1974. 771 p.
Jiménez-Reyes M, Almazán-Sánchez PT, Solache-Ríosa M. Radioactive waste treatments by using zeolites. A short review. J Environ Radioact. 2021;233:106610.doi:10.1016/j.jenvrad.2021.106610
Rachkova NG, Taskaev AI. Immobilization of U, Ra, and Th compounds with analcime-containing rock and hydrolysis lignin. Radiochem. 2011;53:314–321. doi:10.1134/S1066362211030155
Hegazy EZ, Abd El Maksod IH, Abo El Enin RMM. Preparation and characterization of Ti and V modified analcime from local kaolin. Appl Clay Sci. 2010;49:149–155. doi:10.1016/j.clay.2010.04.019
Vereshchagina TA, Kutikhina EA, Chernykh YaYu, Fomenko EV, Mazurova EV, Vereshchagin SN, Bondarenko GN. Preparation and properties of Zr-bearing sorption materials based on coal fly ash microspheres. J Sib Fed Univ Chem. 2019;12:347–363. doi:10.17516/1998-2836-0132
Ames LL. Cation exchange properties of wairakite and anal-cime. Amer Miner. 1966;51:903–909.
Redkin AF, Hemley JJ. Experimental Cs and Sr sorption on analcime in rock-buffered systems at 250–300 °C and Psat and the thermodynamic evaluation of mineral solubilities and phase relations. Eur J Mineral. 2000;12:999–1014. doi:10.1127/0935-1221/2000/0012-0999
Trachenko K, Understanding resistance to amorphization by radiation damage. J Phys Condens Matter. 2004;16:R1491–R1515. doi:10.1088/0953-8984/16/49/R03
Lee WE, Ojovan MI, Stennett MC, Hyatt NC. Immobilisation of radioactive waste in glasses, glass composite materials and ceramics. Adv Appl Ceram. 2006;105:3–12. doi:10.1179/174367606X81669
Luca V, Griffith CS, Drabarek E, Chronis H. Tungsten bronze-based nuclear waste form ceramics. Part 1. Conversion of microporous tungstates to leach resistant ceramics. J Nucl Mater. 2006;358:139–150. doi:10.1016/j.jnucmat.2006.06.017
Borade RB, Clearfield A. Hydrothermal synthesis of an iron silicate with layered structure. Chem Commun. 1997:277–278. doi:10.1039/A606343H
Liu L, Wang S, Zhang B, Jiang G, Yang J. Supercritical hydrothermal synthesis of nano-ZrO2: Influence of technological parameters and mechanism. J Alloys Comp. 2022;898:162878. doi:10.1016/j.jallcom.2021.162878
Zhang M, Sheng X, Zhang Y, Zhou Y, Zhao S, Fu X, Zhang H. Zirconium incorporated micro/mesoporous silica solid acid catalysts for alkylation of o-xylene with styrene. J Porous Mater. 2017;24:109–120. doi:10.1007/s10934-016-0243-7
Luo X, Wang X, Bao S, Liu X, Zhang W, Fang T. Adsorption of phosphate in water using one-step synthesized zirconium-loaded reduced graphene oxide. Sci Rep. 2016;6:39108. doi:10.1038/srep39108
Vereshchagina TA, Kutikhina EA, Solovyov LA, Vereshchagin SN, Mazurova EV, Chernykh YaYu, Anshits AG. Synthesis and structure of analcime and analcime-zirconia composite derived from coal fly ash cenospheres. Microporous Mesoporous Mater. 2018;258:228–235.doi:10.1016/j.micromeso.2017.09.011
Orlova AI, Ojovan MI. Ceramic mineral waste-forms for nuclear waste immobilization. Mater. 2019;12:2638. doi:10.3390/ma12162638
The National Academies Press. Waste Forms Technology and Performance: Final Report. Committee on Waste Forms Technology and Performance. National Research Council: Washington, DC, USA. 2011. 308 p. doi:10.17226/13100
Hamoud MA, Allan KF, Sanad WA, El-Hamouly SH, Ayoub RR. Gamma irradiation induced preparation of poly(acrylamide-itaconic acid)/zirconium hydrous oxide for removal of Cs-134 radionuclide and methylene blue. J Radioanal Nucl Chem. 2014;302:169–178. doi:10.1007/s10967-014-3206-y
Tel H, Altas Y, Gur F, Ugur A. Sorption kinetics of cesium on ZrO2 and ZrO2–SiO2–TiO2 microspheres. Radiochim Acta. 2010;98:215–219. doi:10.1524/ract.2010.1707
Venkatesan KA, Selvam GP, Rao PRV. Sorption of strontium on hydrous zirconium oxide. Sep Sci Technol. 2000;35:2343–2357. doi:10.1081/SS-100102106
Anshits NN, Mikhailova OA, Salanov AN, Anshits AG. Chemical composition and structure of the shell of fly ash non-perforated cenospheres produced from the combustion of the Kuznetsk coal (Russia). Fuel. 2010; 89: 1849−1862. doi:10.1016/j.fuel.2010.03.049
Fomenko EV, Anshits NN, Solovyov LA, Mikhaylova OA, Anshits AG. Composition and morphology of fly ash cenospheres produced from the combustion of Kuznetsk coal. Energy Fuels. 2013; 27: 5440–5448. doi:10.1021/ef400754c
Vereshchagina TA, Kutikhina EA, Chernykh YaYu, Solovyov LA, Zhizhaev AM, Vereshchagin SN, Anshits AG. One-step immobilization of cesium and strontium from alkaline solutions via a facile hydrothermal route. J Nucl Mater. 2018;510:243–255. doi:10.1016/j.jnucmat.2018.08.015
Cements and materials for cement production. Chemical analysis methods. State Standard (GOST) No. 5382–2019. Moscow: IPK, Izdatel’stvo standartov; 2002. 70 p.
Greg SJ, Singh KSW. Adsorption, Surface Area, and Porosity. London: Academic Press; 1982. 304 p.
Wise WS. Handbook of Natural Zeolites. ed. C. Colella. International Zeolite Association. Napoli, Italy, A. De Frede Edotore: Natural Zeolites Commission; 2013. 126 p.
Mu W, Zhang R, Li X, Xie X, Yu Q, Lv K, Wei H, Jian Y. Pyrochlore Ta-doped antimony oxide as a novel adsorbent for efficient strontium removal. RSC Adv. 2015;5:10378–10385. doi:10.1039/C4RA13992E
Shavinsky BM, Levchenko LM, Mitkin VN. Obtaining hydrated antimony pentoxide for the sorption of cesium and strontium ions. Chem Sustain Develop. 2010;18:663–667.
Popa K, Pavela CC. Radioactive wastewaters purification using titanosilicates materials: State of the art and perspectives. Desalination. 2012;293:78–86.doi:10.1016/j.desal.2012.02.027
Bortun AI, Bortun LN, Clearfield A. Hydrothermal synthesis of sodium zirconium silicates and characterization of their properties. Chem Mater. 1997;9:1854–1864. doi:10.1021/cm9701419
Milyutin VV, Nekrasova NA, Kaptakov VO. Modern sorption materials for cesium and strontium radionuclide extraction from liquid radioactive waste radioactive waste. Radioact Waste. 2020;4(13):80—89 (In Russian). doi:10.25283/2587-9707-2020-4-80-89
Misak NZ. Outlines of the ion exchange characteristics of hydrous oxides. Adv Colloid Interface Sci. 1994;51:29–135. doi:10.1016/0001-8686(94)80034-0
Amphlett CB. Inorganic Ion exchangers. New York, London: Elsevier Pub. Co; 1964. 141 p.
Suvorova VA, Kotel’nikov AR, Akhmedzhanova GM. Phase Transformation of Zeolites Saturated with Alkali and Alkaline-Earth Elements into Ceramic. Vestn Ross Akad Nauk Ser Earth Sci. 2002;1:20.
Kotel’nikov AR, Bychkov AM, Zyryanov VN, Akhmedzhanova GM, Gavlina OT. Phase Transformation of Zeolites into Feldspar as a Method for Preparing Aluminosilicate Matrices for Radionuclide Fixation. Geokhimiya. 1995;10:1527–1532.
DOI: https://doi.org/10.15826/chimtech.2022.9.4.18
Copyright (c) 2022 Tatiana A. Vereshchagina, Ekaterina A. Kutikhina, Olga V. Buyko, Alexander G. Anshits

This work is licensed under a Creative Commons Attribution 4.0 International License.
Chimica Techno Acta, 2014–2025
eISSN 2411-1414
Copyright Notice







