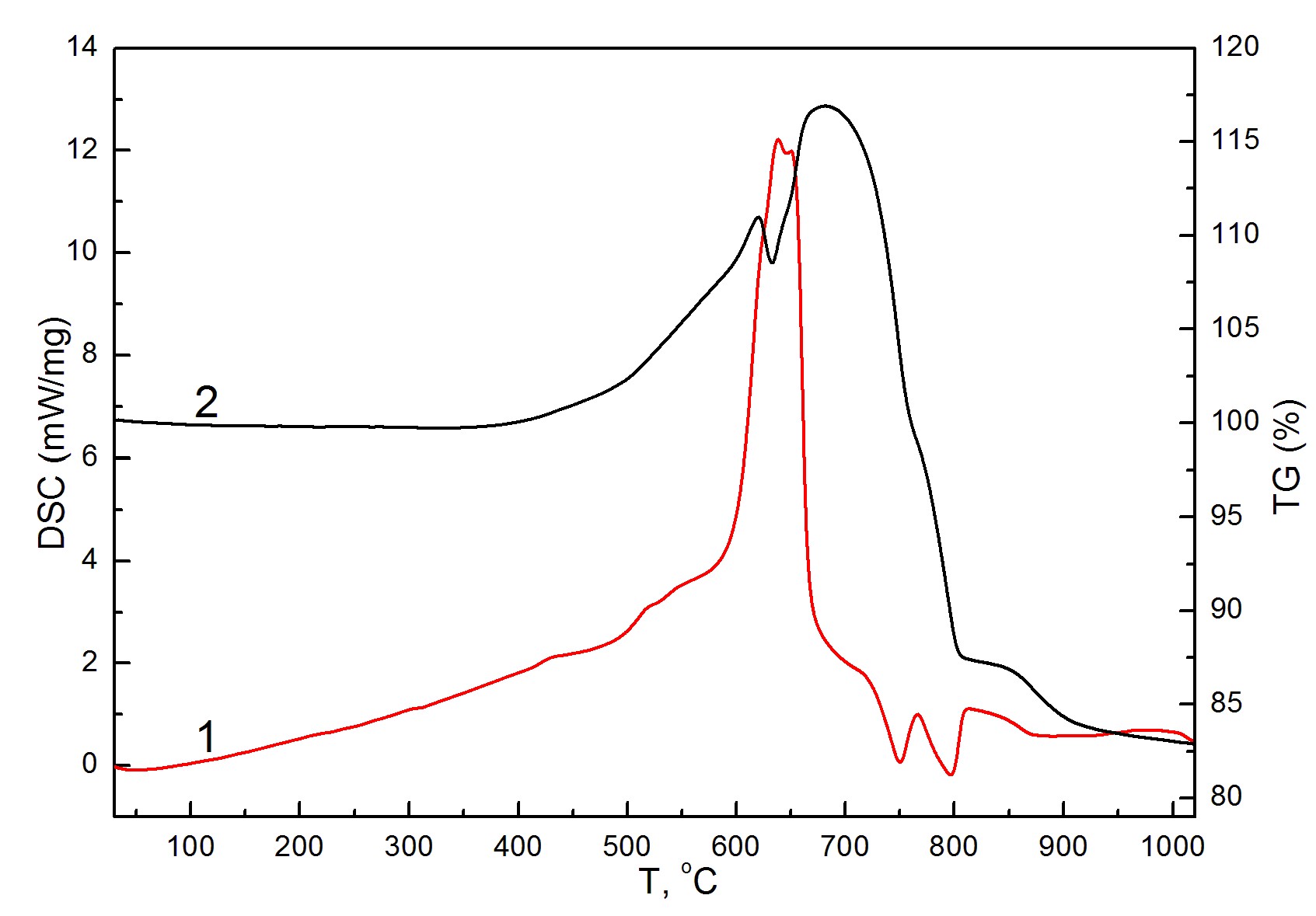
Effect of gas-chromatography column regeneration during the CHN/S analysis of copper-chromium disulfide
Abstract
Keywords
Full Text:
PDFReferences
Krasilnikova AA, Shestopalov MA, Brylev KA, Kirilova IA, Khripko OP, Zubareva KE, Khripko YI, Podorognaya VT, Shestopalova LV, Fedorov VE, Mironov YV. Prospects of molybdenum and rhenium octahedral cluster complexes as X-ray contrast agents. J Inorg Biochem. 2015;144:13–17. doi:10.1016/j.jinorgbio.2014.12.016
Arif M, Li Y, El-Dalatony MM, Zhang C, Li X, Salama E-S. A complete characterization of microalgal biomass through FTIR/TGA/CHNS analysis: An approach for biofuel generation and nutrients removal. Renew Energy. 2021;163:1973–1982. doi:10.1016/j.renene.2020.10.066
Singha G, Saroa A, Khullar S, Mandal SK. Schiff bases of N-(2-aminoethyl)-3-aminopropyltrimethoxysilane and its silatranes: Synthesis and characterization. J Chem Sci. 2015;127(4):679–685. doi:10.1007/s12039-015-0822-1
Litvinova YM, Kuratieva NV, Gayfulin YM, Mironov YV. Structure of ionic cluster complex (PhenH)4[Re4Te4(CN)12]·4H2O. J Struct Chem. 2015;56:1220–1222. doi:10.1134/S0022476615060359
Danial R, Sobri S, Abdullah LC, Mobarekeh MN. FTIR, CHNS and XRD analyses define mechanism of glyphosate herbicide removal by electrocoagulation. Chemosphere. 2019;233:559–569. doi:10.1016/j.chemosphere.2019.06.010
Semenova OI, Yudanova ES, Yeryukov NA, Zhivodkov YA, Sha-mirzaev TS, Maximovskiy EA, Gromilov SA, Troitskaia IB. Per-ovskite CH3NH3PbI3 crystals and films. Synthesis and charac-terization. J Cryst Growth. 2017;462:45–49. doi:10.1016/j.jcrysgro.2017.01.019
Siutkina AI, Makhmudov RR, Shipilovskikh DA. Synthesis and analgesic activity evaluation of derivatives of 2-[(1,4-dioxo-1-amino-4-arylbutyl-2-en-2-yl)amino]-4,5,6,7- tetrahydroben-zo[b]thiophene-3-carboxylic acid. Chim Techno Acta. 2021;8(4):20218404. doi:10.15826/chimtech.2021.8.4.04
Ranjbar ZR, Morsali A. Synthesis, experimental and theoretical studies of two cocrystals in 1:1 stoichiometric ratio from 4,4-bithiazole-2,2-diamine with two hydrogen acceptor molecules. J Chem Sci. 2015;127(11):2015–2021. doi:10.1007/s12039-015-0972-1
Danial R, Sobri S, Abdullah LC, Mobarekeh MN. FTIR, CHNS and XRD analyses define mechanism of glyphosate herbicide removal by electrocoagulation. Chemosphere. 2019;233:559-569. doi:10.1016/j.chemosphere.2019.06.010
Santos ZM, Caroni ALPF, Pereira MR, Silva DR, Fonseca JLC. Determination of deacetylation degree of chitosan: a comparison between conductometric titration and CHN elemental analysis. Carbohydr Res. 2009;344:2591–2595. doi:10.1016/j.carres.2009.08.030
Korotaev EV, Syrokvashin MM, Filatova IYu, Pelmenev KG, Zvereva VV, Peregudova NN. Seebeck coefficient of cation-substituted disulfides CuCr1−xFexS2 and Cu1−xFexCrS2. J Electron Mater. 2018;47:3392. doi:10.1007/s11664-018-6230-9
Korotaev EV, Syrokvashin MM, Filatova IY, Sotnikov AV. Effect of the order-disorder transition on the electronic structure and physical properties of layered CuCrS2. Mater. 2021;14:2729. doi:10.3390/ma14112729
Karmakar A, Dey K, Chatterjee S, Majumdar S, Giri S. Spin correlated dielectric memory and rejuvenation in multiferroic CuCrS2. Appl Phys Lett. 2014;104:052906. doi:10.1063/1.4863937
Boutbila MA, Rasneur J, El Aatmani M, Lyahyaoui H. Point defects in the ternary sulfide CuCrS2. J Alloys Compd. 1996;244:23–26. doi:10.1016/S0925-8388(96)02418-8
Chen Y-X, Zhang B-P, Ge Z-H, Shang P-P. Preparation and thermoelectric properties of ternary superionic conductor Cu-CrS2. J Solid State Chem. 2012;186:109–115. doi:10.1016/j.jssc.2011.11.040
Korotaev EV, Syrokvashin MM, Filatova IY, Zvereva VV. Vanadium doped layered copper-chromium sulfides: The correlation between the magnetic properties and XES data. Vacuum. 2020;179:109390. doi:10.1016/j.vacuum.2020.109390
Ohta M, Hirai S, Kato H, Sokolov VV, Bakovets VV. Thermal Decomposition of NH4SCN for preparation of Ln2S3 (Ln=La and Gd) by sulfurization. Mater Trans. 2009;50:1885–1889. doi:10.2320/matertrans.M2009060
Koshcheeva OS, Zubareva AP, Saprykin AI. CHN analysis of functional materials and their precursors. J Struct Chem. 2010;51:175–178. doi:10.1007/s10947-010-0209-6
DOI: https://doi.org/10.15826/chimtech.2022.9.4.23
Copyright (c) 2022 Irina B. Troitskaia, Mikhail M. Syrokvashin, Evgeny V. Korotaev, Anatoly I. Saprykin

This work is licensed under a Creative Commons Attribution 4.0 International License.
Chimica Techno Acta, 2014–2025
eISSN 2411-1414
Copyright Notice







