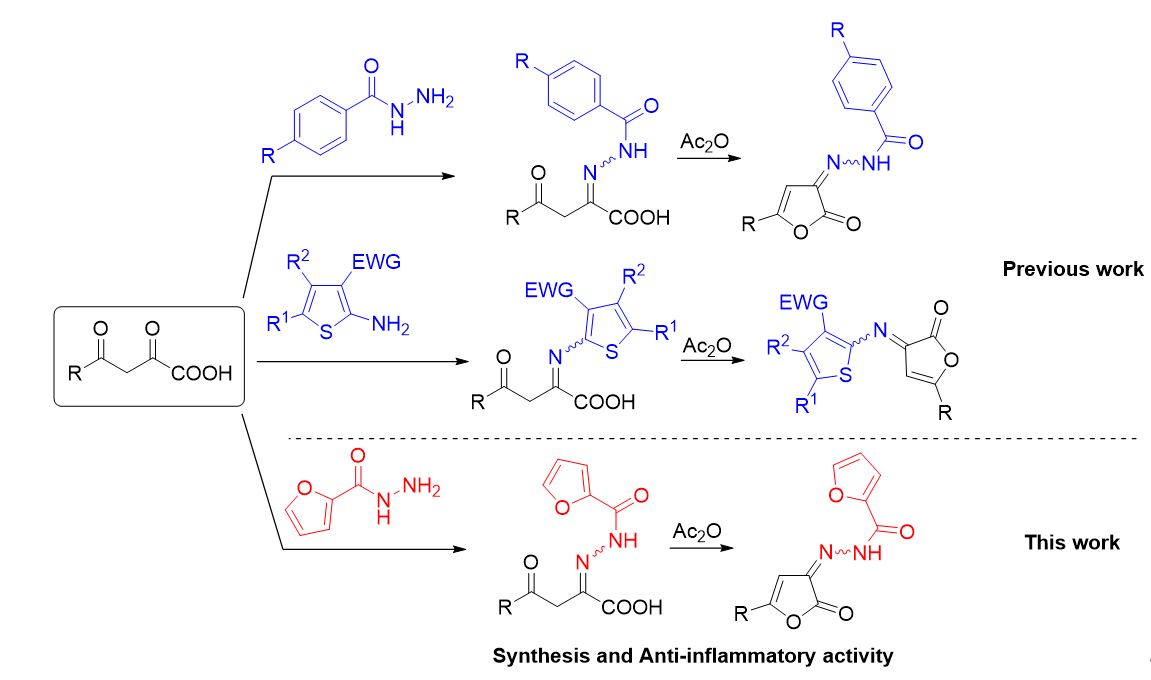
Synthesis, intramolecular cyclization and anti-inflammatory activity of substituted 2-(2-(Furan-2-carbonyl)hydrazono)-4-oxobutanoic Acids
Abstract
Keywords
Full Text:
PDFReferences
Bouz G, Dolezal M. Advances in Antifungal Drug Development: An Up-To-Date Mini Review. Pharmaceutic. 2021;14(12):1312. doi:10.3390/ph14121312
Huang L, Yang J, Wang T, Gao J, Xu D. Engineering of small-molecule lipidic prodrugs as novel nanomedicines for enhanced drug delivery. Nanobiotechnol. 2022;20(1):49. doi:10.1186/s12951-022-01257-4
Jhinjharia D, Kaushik AC, Sahi S. Chapter 3 – Advances in structure-based drug design. Bioinform Pharm Sci. 2021:55. doi:10.1016/B978-0-12-821748-1.00009-9
Samy KE, Gampe C. Medicinal chemistry strategies to extend duration of action of inhaled drugs for intracellular targets. Bioorg Med Chem Lett. 2022;62:128627. doi:10.1016/j.bmcl.2022.128627
Zhao R, Fu J, Zhu L, Chen Y, Liu B. Designing strategies of small-molecule compounds for modulating non-coding RNAs in cancer therapy. J Hematol Oncol. 2022;15(1):14. doi:10.1186/s13045-022-01230-6
Ivashchenko AA, Mitkin OD, Jones JC, Nikitin AV, Koryakova AG, Ryakhovskiy A, Karapetian RN, Kravchenko DV, Aladinskiy V, Leneva IA, Falynskova IN, Glubokova EA. Non-rigid diarylmethyl analogs of baloxavir as cap-dependent endonuclease inhibitors of influenza viruses. J Med Chem. 2020;63:9403. doi:10.1021/acs.jmedchem.0c00565
Babushkina AA, Dogadina AV, Egorov DM, Piterskaia JL, Shtro AA, Nikolaeva YV, Galochkina AV, Kornev AA, Boitsov VM. Efficient synthesis and evaluation of antiviral and antitumor activity of novel 3-phosphonylated thiazolo[3,2-a]oxopyrimidines. Med Chem Res. 2021;30:2203–2215. doi:10.1007/s00044-021-02801-x
Mayorova OA, Yegorova AY. 13C and 1H NMR study of azo coupling products from diazonium salts and furan-2-(3H)-ones. Magn Res Chem. 2015;10:853. doi:10.1002/mrc.4270
Igidov SN, Turyshev AYu, Makhmudov RR, Shipilovskikh DA, Igidov NM, Shipilovskikh SA. Synthesis, Intramolecular Cyclization, and Analgesic Activity of Substituted 2-[2-(Furancarbonyl)hydrazinylydene]-4-oxobutanoic Acids. Russ J Gen Chem. 2022;92(9):1629–1636. doi:10.1134/S1070363222090067
Gavkus DN, Maiorova OA, Borisov MY, Egorova AY. Azo coupling of 5-substituted furan-2(3H)-ones and 1H-pyrrol-2(3H)-ones with arene(hetarene)diazonium salts. Russ J Org Chem. 2012;48:1229–1232. doi:10.1134/s107042801209014x
Ali A, Khalid M, Abid S, Tahir MN, Iqbal J, Ashfaq M, Kanwal F, Lu C, Rehman MF. Green synthesis, SC-XRD, non-covalent interactive potential and electronic communication via DFT exploration of pyridine-based hydrazone. Crystals. 2020;10(9):778. doi:10.3390/cryst10090778
Khalid M, Ali A, Abid S, Tahir MN, Khan MU, Ashfaq M, Imran M, Ahmad A. Facile ultrasound-based synthesis, sc-xrd, dft exploration of the substituted acyl-hydrazones: an experimental and theoretical slant towards supramolecular chemistry. Chem Sel. 2020;5(47):14844–14856. doi:10.1002/slct.202003589
Gorbunova IA, Sharavyeva YuO, Makhmudov RR, Shipilovskikh DA, Shadrin VM, Pulina NA, Shipilovskikh SA. Synthesis and antinociceptive activity of substituted 2-(3-cyano-4,5,6,7-tetrahydrobenzo[b]thiophene-2-ylamino)-4-oxobut-2-enoates. Russ J Gen Chem. 2022;92(10):1–8. doi:10.1134/S1070363222100048
Shipilovskikh SA, Rubtsov AE. One-Pot Synthesis of Thieno[3,2-e]pyrrolo[1,2-a]pyrimidine Derivative Scaffold: A Valuable Source of PARP-1 Inhibitors. J Org Chem. 2019;84:15788. doi:10.1021/acs.joc.9b00711
Sayed HH, Hashem AI, Yousif NM, ElSayed WA. Conversion of 3-Arylazo-5-phenyl-2(3H)-furanones into other heterocycles of anticipated biological activity. Arch Pharm. 2007;6:315. doi:10.1002/ardp.200700043
Siutkina AI, Sharavyeva YO, Chashchina SV, Shipilovskikh SA, Igidov NM. Synthesis and anti-inflammatory activity of N′-substituted 2-[2-(diarylmethylene)hydrazinyl]-5,5-dimethyl-4-oxohex-2-enehydrazides. Russ Chem Bull. 2022;71:496–501. doi:10.1007/s11172-022-3439-9
Kargar H, Fallah-Mehrjardi M, Behjatmanesh-Ardakani R, Munawar KS, Ashfaq M, Tahir MN. Diverse coordination of isoniazid hydrazone Schiff base ligand towards iron(III): Synthesis, characterization, SC-XRD, HSA, QTAIM, MEP, NCI, NBO and DFT study. J Mol Struct. 2022;1250(2):131691. doi:10.1016/j.molstruc.2021.131691
Fernández-García Y, Horst S, Bassetto M, Brancale A, Neyts J, Rogolino D, Sechi M, Carcelli M, Günther S, Rocha-Pereira J. Diketo acids inhibit the cap-snatching endonuclease of several Bunyavirales. Antivir Res. 2020;183:104947. doi:10.1016/j.antiviral.2020.104947
Joksimović N, Janković N, Davidović G, Bugarčić Z. 2,4-Diketo esters: Crucial intermediates for drug discovery. Bioorg Chem. 2020;105:104343. doi:10.1016/j.bioorg.2020.104343
Nair V, Okello M. Integrase Inhibitor Prodrugs: Approaches to Enhancing the Anti-HIV Activity of β-Diketo Acids. Molecules. 2015;20:12623. doi:10.3390/molecules200712623
Sharma H, Sanchez TW, Neamati N, Detorio M, Schinazi RF, Cheng X, Buolamwini JK. Synthesis, docking, and biological studies of phenanthrene β-diketo acids as novel HIV-1 integrase inhibitors. Bioorg Med Chem Lett. 2013;23:6146. doi:10.1016/j.bmcl.2013.09.009
Bobrovskaya OV, Russkih AA, Yankin AN, Dmitriev MV, Bunev AS, Gein VL. Straightforward synthesis of novel spiroether deriva. Synth. Commun. 2021;51:1731. doi:10.1080/00397911.2021.1903930
Sobin FV, Pulina NA, Lipatnikov KV, Starkova AV, Yushkova TA, Naugol’nykh EA. Synthesis and Hemostatic, Anti-Inflammatory, and Anthelminthic Activity of 2-hydroxy-4-oxo-4-(thien-2-yl)but-2-enoic Acid Derivatives. Pharm Chem J. 2021;54:1003. doi:10.1007/s11094-021-02310-6
Pulina NA, Kuznetsov AS, Krasnova AI, Novikova VV. Synthesis, Antimicrobial Activity, and Behavioral Response Effects of N-Substituted 4-Aryl-2-Hydroxy-4-Oxobut-2-Enoic acid hydrazides and their metal complexes. Pharm Chem J. 2019;53:220. doi:10.1007/s11094-019-01983-4
Gein VL, Zamaraeva TM, Gorgopina EV, Dmitriev MV. A four-component Biginelli reaction: new opportunities for the synthesis of functionalized pyrimidines. Chem Heterocycl Compd. 2020;56:339. doi:10.1007/s10593-020-02665-w
Gein VL, Zamaraeva TM, Buzmakova NA, Rudakova IP, Dmitriev MV. Structure and Analgesic Activity of 13-(N-Aryl(N,N-Diethyl)Aminocarbonyl)-9-Methyl-11-Thioxo-8-Oxa-10,12-Diazatricyclo[7.3.1.02,7]Trideca-2,4,6-Trienes and Their 10-N-Phenyl derivatives. Pharm Chem J. 2018;52:515. doi:10.1007/s11094-018-1851-0
Ashfaq M, Munawar K Shahzad BG, Ali A, Tahir MN, Ahmed G, Ramalingam A, Alam MM, Imran M, Sambandam S, Munir B. Single crystal inspection, Hirshfeld surface investigation and DFT study of a novel derivative of 4-fluoroaniline: 4-((4-fluorophenyl)amino)-4-oxobutanoic acid (BFAOB). J Iran Chem Soc. 2022;19(5):1953–1961. doi:10.1007/s13738-021-02432-4
Denisova EI, Lipin DV, Parkhoma KY., Devyatkin IO, Shipilovskikh DA, Chashchina SV, Makhmudov RR, Igidov NM, Shipilovskikh SA. Synthesis, Intramolecular Cyclization, and Antinociceptive Activity of Substituted 2-[2-(4-Nitrobenzoyl)hydrazinylidene]-4-oxobut-2-enoic Acids. Russ J Org Chem. 2021;57:1955. doi:10.1134/s1070428021120083
Lipin DV, Denisova EI, Devyatkin IО, Okoneshnikova ЕА, Shipilovskikh DA, Makhmudov RR, Igidov NM, Shipilovskikh SA. Synthesis and Antinociceptive Activity of Substituted 5-(het)aryl-3-(4-methylbenzoyl)hydrazono-3H-furan-2-ones. Russ J Gen Chem. 2020;91:809. doi:10.1134/S1070363221120161
Gorbunova IA, Shipilovskikh DA, Rubtsov AE, Shipilovskikh SA. Synthesis and Intramolecular Cyclization of Substituted 4-(Het)aryl-4-oxo-2-thienylaminobut-2-enoic Acids Containing Nitrile Group in the Thiophene Ring. Russ J Gen Chem. 2021;91:1623. doi:10.1134/S1070363221090048
Shipilovskikh SA, Makhmudov RR, Lupach DYu, Pavlov PT, Babushkina EV, Rubtsov AE. Synthesis and analgesic activity of substituted 4-(het)aryl-4-oxo-2-thienylaminobut-2-enoic acids. Pharm Chem J. 2013;47(7):366–370. doi:10.1007/s11094-013-0960-z
Sharavyeva YuO, Siutkina AI, Chashchina SV, Novikova VV, Makhmudov RR, Shipilovskikh SA. Synthesis, analgesic and antimicrobial activity of substituted 2-(3-cyano-4,5,6,7-tetrahydrobenzo[b]thiophen-2-ylamino)-4-oxo-4-phenylbut-2-enoates. Russ Chem Bull. 2022;71(3):538. doi:10.1007/s11172-022-3445-y
Gunina E, Zhestkij N, Bachinin S, Fisenko SP, Shipilovskikh DA, Milichko VA, Shipilovskikh SA. The influence of substitutes on the room temperature photoluminescence of 2-amino-4-oxobut-2-enoic acid molecular crystals. Photonics Nanostruct. 2022;48:100990. doi:10.1016/j.photonics.2021.100990
Zhestkij NA, Gunina EV, Fisenko SP, A.E. Rubtsov AE, Shipilovskikh DA, Milichko VA, Shipilovskikh SA. Synthesis of highly stable luminescent molecular crystals based on (E)-2-((3-(ethoxycarbonyl)-5-methyl-4-phenylthiophen-2-yl)amino)-4-oxo-4-(p-tolyl)but-2-enoic acid. Chimica Techno Acta. 2021;8(4):20218411. doi:10.15826/chimtech.2021.8.4.11
Siutkina AI, Chashchina SV, Kizimova IA, Igidov NM, Makhmudov RR, Shipilovskikh SA. Synthesis and Biological Activity of Substituted 2-[2-(Diphenylmethylene)hydrazinyl]-5,5-dimethyl-4-oxohex-2-enoates. Russ J Org Chem. 2021;57(11):1874. doi:10.1134/S1070428021110105
Mironov AN, Bunatyan ND. Metodicheskie ukazaniya po provedeniyu doklinicheskih issledovanij lekarstvennyh sredstv [Guidelines for conducting preclinical studies of medicines]. Moscow: Grif i K; 2012. 944 p. Russian.
Kizimova IA, Igidov NM, Kiselev MA, Ivanov DV, Syutkina AI. Reactions of N'-[2-Oxo-5-R-furan-3(2H)-ylidene]acylhydrazides with Primary and Secondary Alcohols. Russ J Gen Chem. 2020;90(5):815–821. doi:10.1134/s1070363220050096
Izmerov NF, Sanotskii IV, Sidorov KK. Parametry toksikometrii promyshlennykh yadov pri odnokratnom vozdeistvii [Parameters of Toxicometry of Industrial Poisons at a Single Exposure]. Moscow: Meditsina; 1977. 196 p. Russian.
DOI: https://doi.org/10.15826/chimtech.2023.10.1.02
Copyright (c) 2022 Sergei N. Igidov, Dmitriy V. Lipin, Aleksey Yu. Turyshev, Svetlana V. Chashchina, Daria A. Shipilovskikh, Ol’ga V. Zvereva, Ksenia A. Mitusova, Pavel S. Silaichev, Nazim M. Igidov

This work is licensed under a Creative Commons Attribution 4.0 International License.
Chimica Techno Acta, 2014–2025
eISSN 2411-1414
Copyright Notice







