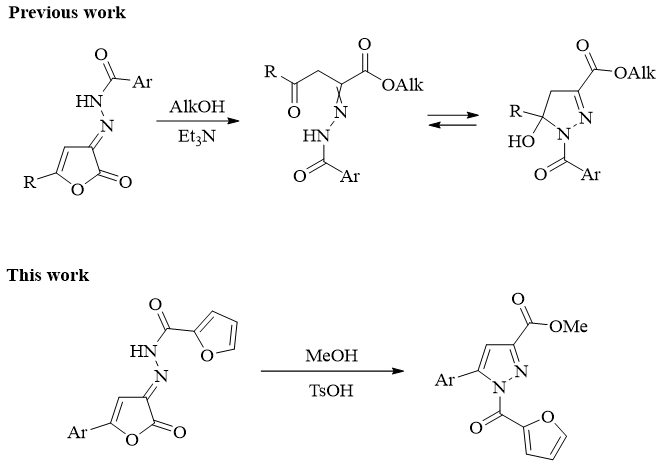
Synthesis, recyclization under the action of methanol and analgetic activity of N'-(5-aryl-2-oxofuran-3(2H)-ylidene)furan-2-carbohydrazides
Abstract
Keywords
Full Text:
PDFReferences
Jhinjharia D, Kaushik AC, Sahi S. Chemoinformatics and Bioinformatics in the Pharmaceutical Sciences. Chapter 3 – Advances in structure-based drug design. Academic Press; 2021. 55–103 p. doi:10.1016/B978-0-12-821748-1.00009-9
Babushkina AA, Dogadina AV, Egorov DM, Piterskaia JL, Shtro AA, Nikolaeva YV, Galochkina AV, Kornev AA, Boitsov VM. Efficient synthesis and evaluation of antiviral and antitumor activity of novel 3-phosphonylated thiazolo[3,2-a]oxopyrimidines. Med Chem Res. 2021;30:2203–2215. doi:10.1007/s00044-021-02801-x
Bouz G, Dolezal M. Advances in Antifungal Drug Development: An Up-To-Date Mini Review. Pharmaceutic (Basel). 2021;14(12):1312. doi:10.3390/ph14121312
Huang L, Yang J, Wang T, Gao J, Xu D. Engineering of small-molecule lipidic prodrugs as novel nanomedicines for enhanced drug delivery. J Nanobiotechnol. 2022;20(1):1–15. doi:10.1186/s12951-022-01257-4
Ivashchenko AA, Mitkin OD, Jones JC, Nikitin AV, Koryakova AG, Ryakhovskiy A, Karapetian RN, Kravchenko DV, Aladinskiy V, Leneva IA, Falynskova IN, Glubokova EA, Govorkova EA, Ivachtchenko AV. Non-rigid Diarylmethyl Analogs of Baloxavir as Cap-Dependent Endonuclease Inhibitors of Influenza Viruses. J Med Chem. 2020;63(17):9403–9420. doi:10.1021/acs.jmedchem.0c00565
Samy KE, Gampe C. Medicinal chemistry strategies to extend duration of action of inhaled drugs for intracellular targets. Bioorg Med Chem Lett. 2022;62:128627. doi:10.1016/j.bmcl.2022.128627
Zhao R, Fu J, Zhu L, Chen Y, Liu B. Designing strategies of small-molecule compounds for modulating non-coding RNAs in cancer therapy. J Hematol Oncol. 2022;15(1):1–19. doi:10.1186/s13045-022-01230-6
Shipilovskikh SA, Rubtsov AE, Zalesov VV. Chemistry of iminofurans 3. Synthesis and intramolecular cyclization of (Z)-4-aryl-2-[3-(ethoxycarbonyl)-4,5,6,7-tetra- hydrobenzo[b]thiophen-2-ylamino]-4-oxobuten-2-oic acids. Chem Heterocycl Compd. 2009;45(6):658–661. doi:10.1007/s10593-009-0334-3
Gavkus DN, Maiorova, OA, Borisov MYu, Egorova AYu. Azo coupling of 5-substituted furan-2(3H)-ones and 1H-pyrrol-2(3H)-ones with arene(hetarene)diazonium salts. Russ J Org Chem. 2012;48(9):1229–1232. doi:10.1134/s107042801209014x
Mayorova OA, Yegorova AY. 13C and 1H NMR study of azo coupling products from diazonium salts and furan-2-(3H)-ones. Magn Reson Chem. 2015;53:853–856. doi:10.1002/mrc.4270
Sayed HH, Hashem AI, Yousif NM, El-Sayed WA. Conversion of 3-arylazo-5-phenyl-2(3H)-furanones into other heterocycles of anticipated biological activity. Arch Pharm (Weinheim). 2007;340(6):315–319. doi:10.1002/ardp.200700043
Shipilovskikh SA, Rubtsov AE. Chemistry of iminofurans. Recyclization of ethyl 2-[2-oxo-5-phenylfuran-3(2H)-ylideneamino]-4,5,6,7-tetrahydro-1-benzothiophene-3-carboxylate by the action of hydrazines. Russ J Org Chem. 2015;50:1853–1855. doi:10.1134/s1070428014120288
Shipilovskikh SA, Rubtsov AE. Decyclization of-2-[5-(4-chlorophenyl)-2-oxofuran-3(2H)-ylideneamino]-4,5,6,7-tetrahydrobenzo[b]thiophene-3-carboxamide upon treatment with aliphatic alcohols. Russ Chem Bull. 2015;63(9):2205–2207. doi:10.1007/s11172-014-0722-4
Shipilovskikh SA, Rubtsov AE. One-Pot Synthesis of Thieno[3,2-e]pyrrolo[1,2-a]pyrimidine Derivative Scaffold: A Valuable Source of PARP-1 Inhibitors. J Org Chem. 2019;84(24):15788–15796. doi:10.1021/acs.joc.9b00711
Shipilovskikh SA, Shipilovskikh DA, Rubtsov AE. Chemistry of iminofurans. Recyclization of ethyl 2-[2-oxo-5-phenylfuran-3(2H)-ylideneamino]-4,5,6,7-tetrahydro-1-benzothiophene-3-carboxylate in reaction with amines. Russ J Org Chem. 2017;53(1):137–140. doi:10.1134/s1070428017010274
Siutkina AI, Sharavyeva YO, Chashchina SV, Shipilovskikh SA, Igidov NM. Synthesis and anti-inflammatory activity of N′-substituted 2-[2-(diarylmethylene)hydrazinyl]-5,5-dimethyl-4-oxohex-2-enehydrazides. Russ Chem Bull. 2022;71(3):496–501. doi:10.1007/s11172-022-3439-9
Siutkina AI, Igidov NM, Kizimova IA. Synthesis and Properties of Alkyl 2-[2-(Diarylmethylidene)hydrazinyl]-5,5-dimethyl-4-oxohex-2-enoates. Russ J Org Chem. 2020;56(4):649–653. doi:10.1134/S1070428020040132
Shipilovskikh SA, Rubtsov AE. Recyclization of 3-(Thiophen-2-yl)imino-3H-furan-2-ones under the Action of Cyanoacetic Acid Derivatives. Russ J Gen Chem. 2020;90(5):809–814. doi:10.1134/S1070363220050084
Vasileva AYu, Shipilovskikh SA, Rubtsov AE. Chemistry of Iminofurans: XV. Decyclization of Ethyl 2-[5-Aryl-2-oxofuran-3(2H)-ylideneamino]-4,5,6,7-tetrahydro-1-benzothiophene-3-carboxylates by the Action of Secondary Amines. Russ J Org Chem. 2018;54(4):582–587. doi:10.1134/S1070428018040115
Shipilovskikh SA, Rubtsov AE. Iminofurans chemistry. Decyclization of ethyl 2-[2-oxo-5-phenylfuran-3(2H)-ylideneamino]-4,5,6,7-tetrahydrobenzo[b]thiophene-3-carboxylate under the action of aliphatic amines. Russ J Org Chem. 2014;50(2):298–300. doi:10.1134/s1070428014020286
Pulina NA, Zalesov VV, Bystritskaya OA, Rubtsov AE, Kutkovaya NV. Synthesis and biological activity of substituted 4-aryl-2-methylenehydrazino-4-oxobut-2-enoic acids and their derivatives. Pharm Chem J. 2009;43(8):444–447. doi:10.1007/s11094-009-0334-8
Bobrovskaya OV, Russkih AA, Yankin AN, Dmitriev MV, Bunev AS, Gein VL. Straightforward synthesis of novel spiroether derivatives. Synth Commun. 2021;51(11):1731–1741. doi:10.1080/00397911.2021.1903930
Sobin FV, Pulina NA, Lipatnikov KV, Starkova AV, Yushkova TA, Naugol’nykh EA. Synthesis and Hemostatic, Anti-Inflammatory, and Anthelminthic Activity of 2-hydroxy-4-oxo-4-(thien-2-yl)but-2-enoic Acid Derivatives. Pharm Chem J. 2021;54(10): 1003–1007. doi:10.1007/s11094-021-02310-6
Fernandez-Garcia Y, Horst ST, Bassetto M, Brancale A, Neyts J, Rogolino D, Sechi M, Carcelli M, Gunther S, Rocha-Pereira J. Diketo acids inhibit the cap-snatching endonuclease of several Bunyavirales. Antiviral Res. 2020;183:104947. doi:10.1016/j.antiviral.2020.104947
Gein ON, Zamaraeva TM, Gein VL. Assessment of the Acute Toxicity and Analgesic Activity of Ethyl-6-Amino-4-Aryl-5-Cyano-2,4-Dihydropyrano-2,3-C]-Pyrazole-3-Carboxylates. Pharm Chem J. 2019;53(1):40–42. doi:10.1007/s11094-019-01952-x
Gein VL, Zamaraeva TM, Buzmakova NA, Rudakova IP, Dmitriev MV. Structure and Analgesic Activity of 13-(N-Aryl(N,N-Diethyl)Aminocarbonyl)-9-Methyl-11-Thioxo-8-Oxa-10,12-Diazatricyclo [7.3.1.02,7]Trideca-2,4,6-Trienes and Their 10-N-Phenyl Derivatives. Pharm Chem J. 2018;52(6):515–517. doi:10.1007/s11094-018-1851-0
Gein VL, Zamaraeva TM, Gorgopina EV, Dmitriev MV. A four-component Biginelli reaction: new opportunities for the synthesis of functionalized pyrimidines. Chem Heterocycl Compd. 2020;56(3):339–346. doi:10.1007/s10593-020-02665-w
Joksimovic N, Jankovic N, Davidovic G, Bugarcic Z. 2,4-Diketo esters: Crucial intermediates for drug discovery. Bioorg Chem. 2020;105:104343. doi:10.1016/j.bioorg.2020.104343
Nair V, Okello 10.1016/j.bmcl.2013.09.009 M. Integrase Inhibitor Prodrugs: Approaches to Enhancing the Anti-HIV Activity of beta-Diketo Acids. Molecules. 2015;20(7):12623–12651. doi:10.3390/molecules200712623
Pulina NA, Kuznetsov AS, Krasnova AI, Novikova VV. Synthesis, Antimicrobial Activity, and Behavioral Response Effects of N-Substituted 4-Aryl-2-Hydroxy-4-Oxobut-2-Enoic Acid Hydrazides and Their Metal Complexes. Pharm Chem J. 2019;53(3):220–224. doi:10.1007/s11094-019-01983-4
Sharma H, Sanchez TW, Neamati N, Detorio M, Schinazi RF, Cheng X, Buolamwini JK. Synthesis, docking, and biological studies of phenanthrene beta-diketo acids as novel HIV-1 integrase inhibitors. Bioorg Med Chem Lett. 2013;23(22):6146–6151. doi:10.1016/j.bmcl.2013.09.009
Siutkina AI, Chashchina SV, Makhmudov RR, Kizimova IA, Shipilovskikh SA, Igidov NM. Synthesis and Biological Activity of Substituted 2-[2-(Diphenylmethylene)hydrazinyl]-5,5-dimethyl-4-oxohex-2-enoates. Russ J Org Chem. 2021;57(11):1874–1881. doi:10.1134/s1070428021110105
Cvijetic IN, Verbic TZ, Ernesto de Resende P, Stapleton P, Gibbons S, Juranic IO, Drakulic BJ, Zloh M. Design, synthesis and biological evaluation of novel aryldiketo acids with enhanced antibacterial activity against multidrug resistant bacterial strains. Eur J Med Chem. 2018;143:1474–1488. doi:10.1016/j.ejmech.2017.10.045
Wang Z, Tang J, Salomon CE, Dreis CD, Vince R. Pharmacophore and structure-activity relationships of integrase inhibition within a dual inhibitor scaffold of HIV reverse transcriptase and integrase. Bioorg Med Chem. 2010;18(12):4202–4211. doi:10.1016/j.bmc.2010.05.004
Denisova EI, Lipin DV, Parkhoma KYu, Devyatkin IO, Shipilovskikh DA, Chashchina SV, Makhmudov RR, Igidov NM, Shipilovskikh SA. Synthesis, Intramolecular Cyclization, and Antinociceptive Activity of Substituted 2-[2-(4-Nitrobenzoyl)hydrazinylidene]-4-oxobut-2-enoic Acids. Russ J Org Chem. 2021;57(12):1955–1960. doi:10.1134/s1070428021120083
Lipin DV, Denisova EI, Devyatkin IО, Okoneshnikova ЕА, Shipilovskikh DA, Makhmudov RR, Igidov NM, Shipilovskikh SA. Synthesis and Antinociceptive Activity of Substituted 5-(Het)aryl-3-(4-methylbenzoyl)hydrazono-3H-furan-2-ones. Russ J Gen Chem. 2021;91(12):2469–2474. doi:10.1134/s1070363221120161
Babushkina AA, Piterskaya YL, Shtro AA, Nikolaeva YV, Galochkina AV, Klabukov AM, Egorov DM. Synthesis, Phosphonylation, and Anti-Viral Activity of Some 6-Aryl-5-cyano-2-thiouracils. Russ J Gen Chem. 2022;92(1):18–23. doi:10.1134/s1070363222010042
Egorova AV, Egorov DM, Nyanikova GG, Rogatko DA, Garabadzhiu AV. Biocidal Activity of Benzothiazole and Arylaminomalonate Derivatives. Russ J Gen Chem. 2018;87(13):3255–3258. doi:10.1134/s1070363217130217
Gorbunova IA, Shipilovskikh DA, Rubtsov AE, Shipilovskikh SA. Synthesis and Intramolecular Cyclization of Substituted 4-(Het)aryl-4-oxo-2-thienylaminobut-2-enoic Acids Containing Nitrile Group in the Thiophene Ring. Russ J Gen Chem. 2021;91(9):1623–1628. doi:10.1134/s1070363221090048
Shipilovskikh SA, Rubtsov AE. Synthesis of New Substituted 3-(Thien-2-yl)imino-3H-furan-2-ones. Russ J Gen Chem. 2020;90(6):943–7. doi:10.1134/S1070363220060031
Shipilovskikh SA, Rubtsov AE. Synthesis of substituted 2-((2-oxofuran-3(2H)-ylidene)amino)-4,5,6,7-tetrahydrobenzo[b]thiophene-3-carboxamides. AIP Conf Proc 2020;2280(1):030016. doi:10.1063/5.0018486
Denisova EI, Shipilovskikh SA, Makhmudov RR, Rubtsov AE. Search of analgesic activity of N-substituted 2-((3-R-4,5,6,7-tetrahydrobenzo[b]thiophen-2-yl)amino)-4-oxo-4-phenylbut-2-enamides. AIP Conference Proceedings 2020;2280(1):040013. doi:10.1063/5.0018515
Gorbunova IA, Sharavyeva YuO, Makhmudov RR, Shipilovskikh DA, Shadrin VM, Pulina NA, Shipilovskikh SA. Synthesis and Antinociceptive Activity of Substituted 2-(3-Cyano-4,5,6,7-tetrahydrobenzo[b]thiophene-2-ylamino)-4-oxobut-2-enoates. Russ J Gen Chem. 2022;92(10):1–7. doi:10.1134/S1070363222100048
Shipilovskikh SA, Makhmudov RR, Lupach DYu, Pavlov PT, Babushkina EV, Rubtsov AE. Synthesis and Analgesic Activity of Substituted 4-(Het)aryl-4-oxo-2-thienylaminobut-2-enoic Acids. Pharm Chem J. 2013;47(7):366–370. doi:10.1007/s11094-013-0960-z
Nayak SG, Poojary B, Kamat V, Puthran D. Novel thiazolidin‐4‐one clubbed thiophene derivatives via Gewald synthesis as anti‐tubercular and anti‐inflammatory agents. J Chin Chem Soc. 2021;68(6):1116–1127. doi:10.1002/jccs.202000166
Sharavyeva YuO, Siutkina AI, Chashchina SV, Novikova VV, Makhmudov RR, Shipilovskikh SA. Synthesis, analgesic and antimicrobial activity of substituted 2-(3-cyano-4,5,6,7-tetrahydrobenzo[b]thiophen-2-ylamino)-4-oxo-4-phenylbut-2-enoates. Russ Chem Bull. 2022;71(3):538–542. doi:10.1007/s11172-022-3445-y
Fogue PS, Lunga PK, Fondjo ES, De Dieu Tamokou J, Thaddee B, Tsemeugne J, Tchapi AT, Kuiate JR. Substituted 2-aminothiophenes: antifungal activities and effect on Microsporum gypseum protein profile. Mycoses. 2012;55(4):310–317. doi:10.1111/j.1439-0507.2011.02089.x
Shipilovskikh SA, Makhmudov RR, Balandina SY, & Rubtsov AE. Search of antimicrobial activity in a series of substituted 4-aryl-4-oxo-2-tienilaminobut-2-enoic acids. AIP Conference Proceedings 2020;2280(1):030018. doi:10.1063/5.0018494
Vlasova O.D. Krolenko KYu, Nechayev MA, Shynkarenko PE, Kabachnyy VI, Vlasov SV. Efficient method for the synthesis of novel substituted thieno[2,3-d]pyrimidine-4-carboxylic acids, their derivatization, and antimicrobial activity. Chem Heterocycl Comp. 2019;55(2):184–188. doi:10.1007/s10593-019-02437-1
Gunina E, Zhestkij N, Bachinin S, Fisenko SP, Shipilovskikh DA, Milichko VA, Shipilovskikh SA. The influence of substitutes on the room temperature photoluminescence of 2-amino-4-oxobut-2-enoic acid molecular crystals. Photonics Nanostruct. 2022;48:100990. doi:10.1016/j.photonics.2021.100990
Zhestkij NA, Gunina EV, Fisenko SP, Rubtsov AE, Shipilovskikh DA, Milichko VA, Shipilovskikh SA. Synthesis of highly stable luminescent molecular crystals based on (E)-2-((3-(ethoxycarbonyl)-5-methyl-4-phenylthiophen-2-yl)amino)-4-oxo-4-(p-tolyl)but-2-enoic acid. CTA. 2021;8(4). doi:10.15826/chimtech.2021.8.4.11
El‐Mekabaty A, Awad HM. Convenient synthesis of novel sulfonamide derivatives as promising anticancer agents. J Heterocycl Chem. 2019;57(3):1123–1132. doi:10.1002/jhet.3849
Kovtun AV, Tokarieva SV, Varenichenko SA, Farat OK, Mazepa AV, Dotsenko VV, Markov VI. Spirocyclic thienopyrimidines: synthesis, new rearrangement under Vilsmeier conditions and in silico prediction of anticancer activity. Biopolym Cell. 2020;36(4):279. doi:10.7124/bc.000A2C
Shah R, Verma PK. Synthesis of thiophene derivatives and their anti-microbial, antioxidant, anticorrosion and anticancer activity. BMC Chem. 2019;13(1):1–13. doi:10.1186/s13065-019-0569-8
Igidov SN, Turyshev AY, Makhmudov RR, Shipilovskikh DA, Igidov NM, Shipilovskikh SA. Synthesis, Intramolecular Cyclization, and Analgesic Activity of Substituted 2-[2-(Furancarbonyl)hydrazinylydene]-4-oxobutanoic Acids. Russ J Gen Chem. 2022;92(9):1629–1636. doi:10.1134/S1070363222090067
Kizimova IA, Igidov NM, Kiselev M.A, Ivanov DV, Syutkina AI. Reactions of N'-[2-Oxo-5-R-furan-3(2H)-ylidene]acylhydrazides with Primary and Secondary Alcohols. Russ J Gen Chem. 2020;90(5):815–821. doi:10.1134/S1070363220050096
Joly N, Bettoni L, Gaillard S, Poater A, Renaud JL. Phosphine-Free Ruthenium Complex-Catalyzed Synthesis of Mono- or Dialkylated Acyl Hydrazides via the Borrowing Hydrogen Strategy. J Org Chem. 2021;86(9):6813–6825. doi:10.1021/acs.joc.1c00654
Sof’ina OA, Igidov NM, Koz’minykh EN, Trapeznikova NN, Kasatkina YuS, Koz’minykh VO. Reactions of Acylpyruvic Acids and 2,3-Dihydrofuran-2,3-diones with 2,3-Diaminopyridine. Russ J Org Chem. 2001;37(7):1017–1025. doi:10.1023/A:1012438902959
Verbic TZ, Drakulic BJ, Zloh MF, Pecelj JR, Popovic GV, Juranic IO. An LFER study of the protolytic equilibria of 4-aryl-2,4-dioxobutanoic acids in aqueous solutions. J Serb Chem Soc. 2007;72(12):1201–1216. doi:10.2298/JSC0712201V
Eddy NB, Leimbach DJ. Synthetic analgesics. II. Dithienylbutenyl- and dithienylbutylamines. J Pharmacol Exp Ther. 1953;107(3):385–393. https://jpet.aspetjournals.org/content/107/3/385
Mironov AN. Guidelines for conducting preclinical studies of drugs. Part one. M.: Grif and K, 2012. 944 p. Russian.
Belenky ML. Elements of quantitative evaluation of the pharmacological effect. 2nd ed. Leningrad: Medgiz, 1963. 146 p. Russian.
DOI: https://doi.org/10.15826/chimtech.2023.10.1.01
Copyright (c) 2022 Sergei N. Igidov, Irina A. Gorbunova, Aleksey Yu. Turyshev, Daria A. Shipilovskikh, Danil A. Kozlov, Anna S. Rogova, Ramiz R. Makhmudov, Pavel S. Silaichev, Nazim M. Igidov

This work is licensed under a Creative Commons Attribution 4.0 International License.
Chimica Techno Acta, 2014–2025
eISSN 2411-1414
Copyright Notice







