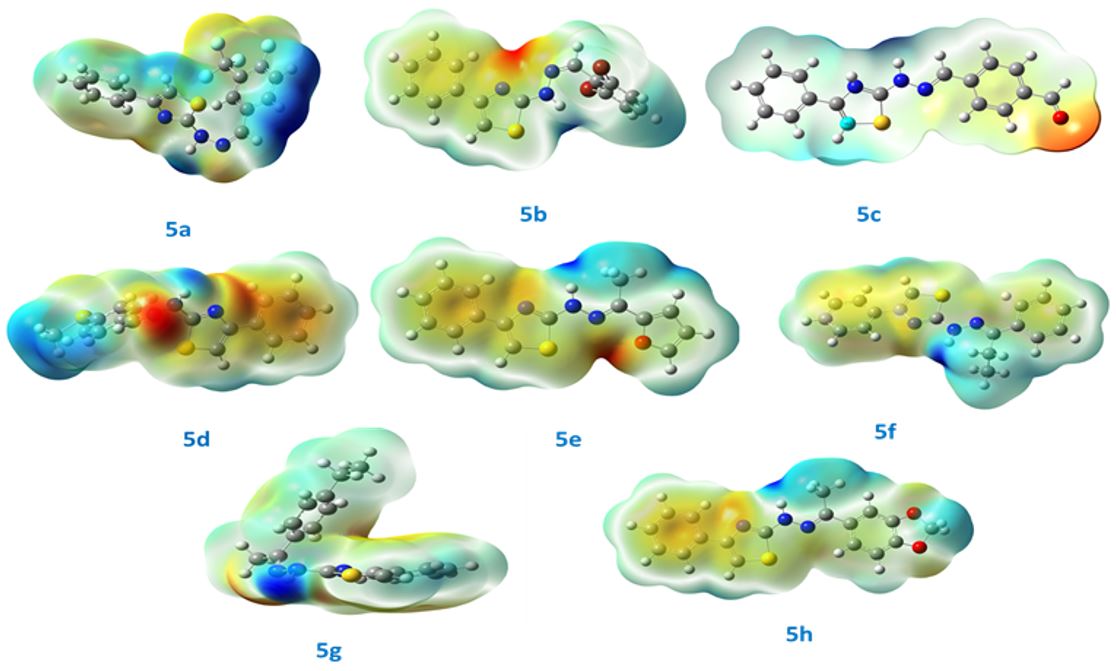
Synthesis, computational study, solvatochromism and biological studies of thiazole-owing hydrazone derivatives
Abstract
Keywords
Full Text:
PDFReferences
Mallikarjuna SM, Padmashali MSMB, Chandrashekharappa S, Sandeep C. Synthesis, anticancer and antituberculosis studies for [1-(4-chlorophenyl) Cyclopropyl](Piperazine-yl) Methanone derivates. Int J Pharm Sci Res. 2014;6(7):423–427.
Nagesh HK, Padmashali B, Sandeep C, Musturappa TE, Lokesh M. Synthesis and characterization of novel benzothiophene substituted oxadiazole derivatives and their antimicrobial activity. Pharma Chem. 2015;7:129–136.
Pattanashetty SH, Hosamani, KM, Barretto DA. Microwave assisted synthesis, computational study and biological evaluation of novel quinolin-2 (1H)-one based pyrazoline hybrids. Chem Data Coll. 2018;15:184–196. doi:10.1016/j.cdc.2018.06.003
Pattanashetty SH, Hosamani KM, Shettar AK, Mohammed Shafeeulla R. Design, synthesis and computational studies of novel carbazole n‐phenylacetamide hybrids as potent antibacterial, anti‐inflammatory, and antioxidant agents. Synth Commun. 2018;55:1765–1774. doi:10.1080/00397911.2019.1620281
Nandeshwarappa BP, Chandrashekharappa S, Ningegowda R, Selenium-containing heterocycles: Synthetic investigation of some new series 3-(5-mercapto-1, 3, 4-oxadiazol-2-yl)-2H-selenopyrano [2, 3-b] quinolin-2-ones. Chem Data Collec. 2020;29:100510. doi:10.1016/j.cdc.2020.100510
Chen L, Liu XY, Zou YX. Recent advances in the construction of phosphorus‐substituted heterocycles. Adv Synth Catal. 2020;362:1724–1818. doi:10.1002/adsc.201901540
Khatoon H, Abdulmalek E. A focused review of synthetic applications of lawesson’s reagent in organic synthesis. Mol. 2021;26:6937. doi:10.3390/molecules26226937
Sharma PC, Bansal KK, Sharma A, Sharma D, Deep A. Thiazole-containing compounds as therapeutic targets for cancer therapy. Eur J Med Chem. 2020;188:112016. doi:10.1016/j.ejmech.2019.112016
Ashwani Kumar, Anup Pandith, Hong-Seok Kim,Pyrene-appended imidazolium probe for 2,4,6-trinitrophenol in water. Sensors Actuators B Chem. 2016;231:293–301. doi:10.1016/j.snb.2016.03.033
Prakashaiah BG, Vinaya Kumara D, Anup Pandith A, Nityananda Shetty A, Amitha Rani BE. Corrosion inhibition of 2024-T3 aluminum alloy in 3.5% NaCl by thiosemicarbazone derivatives. Corros Sci. 2018;136:326–338. doi:10.1016/j.corsci.2018.03.021
Pathania S, Narang RK, Rawal RK. Role of sulphur-heterocycles in medicinal chemistry: An update. Eur J Med Chem. 2019;180:486–508. doi:10.1016/j.ejmech.2019.07.043
Matiadis D, Sagnou M. Pyrazoline hybrids as promising anticancer agents: An up-to-date overview. Int J Mol Sci. 2020;21:5507. doi:10.3390/ijms21155507
Pereira PS, de Lima MDCA, Neto PPM, de Morais Oliveira-Tintino CD, Tintino SR, de AlencarMenezes IR, Silva TG. Thiazolidinedione and thiazole derivatives potentiate norfloxacin activity against NorA efflux pump over expression in Staphylococcus aureus 1199B strains. Bioorg Med Chem. 2019;27:3797–3804. doi:10.1016/j.bmc.2019.07.006
Ankali KN, Rangaswamy J, Shalavadi M, Naik N, naik Krishnamurthy G. Synthesis and molecular docking of novel 1, 3-thiazole derived 1, 2, 3-triazoles and in vivo biological evaluation for their anti anxiety and anti inflammatory activity. J Mol Struct. 2021;123:130357. doi:10.1016/j.molstruc.2021.130357
Gürsoy E, Dincel ED, Naesens L, Güzeldemirci NU. Design and synthesis of novel imidazo [2, 1-b] thiazole derivatives as potent antiviral and antimycobacterial agents. Bioorg Chem. 2020;95:103496. doi:10.1016/j.bioorg.2019.103496
Eissa SI, Farrag AM, Abbas SY, El Shehry MF, Ragab A, Fayed EA, Ammar YA. Novel structural hybrids of quinoline and thiazole moieties: Synthesis and evaluation of antibacterial and antifungal activities with molecular modeling studies. Bioorg Chem. 2021;110:104803. doi:10.1016/j.bioorg.2021.104803
De Santana TI, de Oliveira Barbosa M, de Moraes Gomes PAT, da Cruz ACN, da Silva TG, Leite ACL. Synthesis, anticancer activity and mechanism of action of new thiazole derivatives, Eur J Med Chem. 2018;144;874–886. doi:10.1016/j.ejmech.2017.12.040
Kumar G, Singh NP. Synthesis, anti-inflammatory and analgesic evaluation of thiazole/oxazole substituted benzothiazole derivatives. Bioorg Chem. 2021;107:104608. doi:10.1016/j.bioorg.2020.104608
Eryılmaz S, Çelikoğlu ET, İdil Ö, İnkaya E, Kozak Z, Mısır E, Gül M. Derivatives of pyridine and thiazole hybrid: Synthesis, DFT, biological evaluation via antimicrobial and DNA cleavage activity. Bioorg Chem. 2020;95:103476. doi:10.1016/j.bioorg.2019.103476
Cuevas JM, Seoane-Rivero R, Navarro R, Marcos-Fernández Á. Coumarins into polyurethanes for smart and functional materials. Polym. 2020;12:630. doi:10.3390/polym12030630
Eltyshev AK, Dzhumaniyazov TH, Suntsova PO, Minin AS, Pozdina VA, Dehaen W, Belskaya NP. 3-Aryl-2-(thiazol-2-yl) acrylonitriles assembled with aryl/hetaryl rings: design of the optical properties and application prospects. Dyes Pigment. 2021;184:108836. doi:10.1016/j.dyepig.2020.108836
Godugu K, Shaik S, Pinjari MKM, Gundala TR, Subramanyam DVC, Loka SS, Nallagondu CGR. Solid state thiazole-based fluorophores: Promising materials for white organic light emitting devices. Dyes Pigment. 2021;187:109077. doi:10.1016/j.dyepig.2020.109077
Godugu K, Shaik S, Pinjari MKM, Gundala TR, Subramanyam DVC, Loka SS, Nallagondu CGR. Solid state thiazole-based fluorophores: promising materials for white organic light emitting devices. Dyes Pigment. 2021;187:109077. doi:10.1016/j.dyepig.2020.109077
Nandeshwarappa BP, Chandrashekharappa S, Sadashiv SO. Synthesis and antibacterial evaluation of 3-acetyl-2H-selenopyrano [2, 3-b] quinolin-2-ones. Chem Data Collect. 2020;28:100484–100495. doi:10.1016/j.cdc.2020.100484
Fazil S, Smitha M, Mary YS, Mary YS, Chandramohan V, Kumar N, Van Alsenoy C. Structural (SC-XRD), spectroscopic, DFT, MD investigations and molecular docking studies of a hydrazone derivative. Chem Data Collect. 2020;30:100588. doi:10.1016/j.cdc.2020.100588
Praveen Kumara CH, Katagi Manjunatha S, Nandeshwarappaa BP. Synthesis of novel pyrazolic analogues of chalcones as potential antibacterial and antifungal agents. Curr Chem Lett. 2023;12:1. doi:10.5267/j.ccl.2023.2.001
Tripathi RK, Ayyannan SR. Evaluation of 2-amino-6-nitrobenzothiazole derived hydrazones as acetylcholinesterase inhibitors: in vitro assays, molecular docking and theoretical ADMET prediction. Med Chem Res. 2018;27:709–725. doi:10.1007/s00044-017-2095-3
Abu-Dief AM, El-khatib RM, El Sayed SM, Alzahrani S, Alkhatib F, El-Sarrag G, Ismael M. Tailoring structural elucidation, DFT calculation, DNA interaction and pharmaceutical applications of some aryl hydrazone Mn (II), Cu (II) and Fe (III) complexes. J Mol Struct. 2021;1244:131017. doi:10.1016/j.molstruc.2021.131017
Gamov GA, Zavalishin MN, Petrova MV, Khokhlova AY, Gashnikova AV, Kiselev AN, Sharnin VA. Interaction of pyridoxal-derived hydrazones with anions and Co2+, Co3+, Ni2+, Zn2+ cations. Phys Chem Liquid. 2021;59:666–678. doi:10.1080/00319104.2020.1774878
Frisch MJEA, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, heeseman CJR, Nakatsuji H. gaussian 09, Revision d. 01, Gaussian. Inc., Wallingford CT, 2009; 201 p.
Schlegel HB. Optimization of equilibrium geometries and transition structures. J Comput Chem. 1982;3:214–218. doi:10.1002/jcc.540030212
Ditchfield R, Miller DP, Pople JA. Self‐consistent molecular orbital methods molecular orbital theory of NMR chemical shifts. J Chem Phys. 1971;54:4186–4193. doi:10.1063/1.1674657
Dennington R, Keith T, Millam J, GaussView, version 2009; 5.
Kumar CP, Katagi MS, Nandeshwarappa BP. Novel synthesis of quinoline chalcone derivatives-Design, synthesis, characterization and antimicrobial activity. Chem Data Collect. 2022;42:100955. doi:10.1016/j.cdc.2022.100955
Nandeshwarappa BP, Chandrashekharappa S, Prakash GK. Nitrogen and selenium containing heterocycles: Part-1: Synthesis of some new substituted 3-(5-(2-oxopropylthio)-1, 3, 4-oxadiazol-2-yl)-2H-selenopyrano [2, 3-b] quinolin-2-ones, Chem Data Collect. 2020;29:100534–100541. doi:10.1016/j.cdc.2020.100534
Kenchappa R, Bodke YD, Asha B, Telkar S, Sindhe MA. Synthesis, antimicrobial, and antioxidant activity of benzofuranbarbitone and benzofuranthiobarbitone derivatives. Med Chem Res. 2014;23:3065–3081. doi:10.1007/s00044-013-0892-x
Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays, J I Meth. 1983;65:55–63. doi:10.1016/0022-1759(83)90303-4
Manjunatha B, Bodke YD, Jain R. Novel isoxazolone based azo dyes: synthesis, characterization, computational, solvatochromic UV-Vis absorption and biological studies. J Mol Struct. 2021;1244:130933. doi:10.1016/j.molstruc.2021.130933
Schüttelkopf AW, Van Aalten DM. PRODRG: a tool for high-throughput crystallography of protein–ligand complexes. Acta Crystallograph Sec D Biol Crystallograph. 2004;60:1355–1363. doi:10.1107/S0907444904011679
Manjunatha B, Bodke YD, Kumaraswamy HM, Meghana P, Nagaraja O. Novel thioether linked 4-hydroxycoumarin derivatives: Synthesis, characterization, in vitro pharmacological investigation and molecular docking studies. J Mol Struct. 2022; 1249; 131642. doi:10.1016/j.molstruc.2021.131642
Manjunatha B, Bodke YD, Nagaraja O, Nagaraju G, Sridhar MA. Coumarin-benzothiazole based azo dyes: synthesis, characterization, computational, photophysical and biological studies. J Mol Struct. 2021;1246:131170. doi:10.1016/j.molstruc.2021.131170
Hassan AA, Ibrahim YR, El‐Sheref EM, Abdel‐Aziz M, Bräse S, & Nieger M. Synthesis and Antibacterial Activity of 4‐Aryl‐2‐(1‐substituted ethylidene) thiazoles. Archiv der Pharmazie. 2013;346:562–570. doi:10.1002/ardp.201300099
El-Naggar AM, El-Hashash MA, Elkaeed EB. Eco-friendly sequential one-pot synthesis, molecular docking, and anticancer evaluation of arylidene-hydrazinyl-thiazole derivatives as CDK2 inhibitors. Bioorg Chem. 2015;108:104615. doi:10.1016/j.bioorg.2020.104615
Kirkwood ZI, Millar BC, Downey DG, Moore JE, Antimicrobial effect of dimethyl sulfoxide and N, N-Dimethylformamide on Mycobacterium abscessus: implications for antimicrobial susceptibility testing. Int J Mycobacteriol. 2018;7:134. doi:10.4103/ijmy.ijmy_35_18
Dulian P, Nachit W, Jaglarz J, Zięba P, Kanak J, Żukowski W. Photocatalytic methylene blue degradation on multilayer transparent TiO2 coatings. Opt Mater (Amst). 2019;90:264–272. doi:10.1016/j.optmat.2019.02.041
DOI: https://doi.org/10.15826/chimtech.2023.10.1.10
Copyright (c) 2023 C. Kiran Yadav, B.P. Nandeshwarappa, K.M. Mussuvir Pasha

This work is licensed under a Creative Commons Attribution 4.0 International License.
Chimica Techno Acta, 2014–2025
eISSN 2411-1414
Copyright Notice







