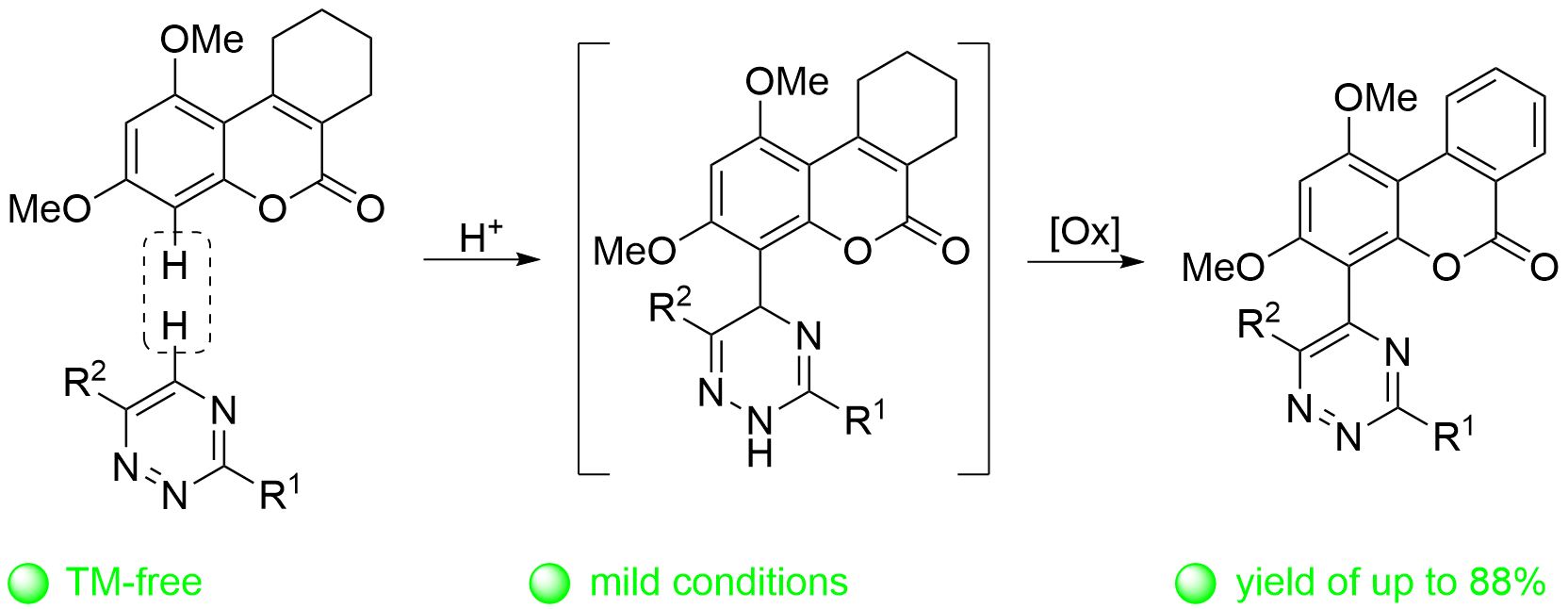
Expedient synthesis of 1,2,4-triazinyl substituted benzo[c]coumarins via double oxidation strategy
Abstract
Keywords
Full Text:
PDFReferences
Fan Y, Wu Y, Hou J, Wang P, Peng X, Ge G. Coumarin-based near-infrared fluorogenic probes: recent advances, challenges and future perspectives. Coord Chem Rev. 2023;480(1):215020. doi:10.1016/j.ccr.2023.215020
Pooja, PH, Aggarwal S, Vats M, Rawat V, Pathak SR. Coumarin-based chemosensors for metal ions detection. Asian J Org Chem. 2022;11(12):e202200455. doi:10.1002/ajoc.202200455
Cao D, Liu Z, Verwilst P, Koo S, Jangjili P, Kim J, Lin W. Coumarin-based small-molecule fluorescent chemosensors. Chem Rev. 2019;119(18):10403–10519. doi:10.1021/acs.chemrev.9b00145
Kim H, Sarkar S, Ahn K. A two-photon ratiometric sensing platform based on solid-state luminescent benzocoumarin: application to prolonged bioimaging of hydrogen peroxide. Chem Asian J. 2022;17(4):e202101317. doi:10.1002/asia.202101317
Sarkar S, Shil A, Nandy M, Singha S, Reo Y, Yang Y, Ahn K. Rationally designed two-photon ratiometric elastase probe for investigating inflammatory bowel disease. Anal Chem. 2022;94(2):1373–1381. doi:10.1021/acs.analchem.1c04646
Sarkar S, Shil A, Jung YL, Singha S, Ahn KH. Rapid point-of-care quantification of human serum albumin in urine based on ratiometric fluorescence signaling driven by intramolecular H-bonding. ACS Sens. 2022;7(12):3790–3799. doi:10.1021/acssensors.2c01684
Sarkar S, Shil A, Nandy M, Singha S, Reo YJ, Yang YJ, Ahn KH. Rationally designed two-photon ratiometric elastase probe for investigating inflammatory bowel disease. Anal Chem. 2022;94(2):1373–1381. doi:10.1021/acs.analchem.1c04646
Sarkar AR, Heo CH, Xu L, Lee HW, Si HY, Byun JW, Kim HM. A ratiometric two-photon probe for quantitative imaging of mitochondrial pH values. Chem Sci. 2016;7:766–773. doi:10.1039/C5SC03708E
Zhang H, Liu X, Gong Y, Yu T, Zhao Y. Synthesis and characterization of SFX-based coumarin derivatives for OLEDs. Dyes Pigments. 2021;185:108969. doi:10.1016/j.dyepig.2020.108969
Kumar N, Udayabhanu, AAA, Mahadevan KM, Nagaraju G. Solvent free and green synthesis of efficient solvochromism based coumarin moieties for quick visualization of LFPs and OLEDs applications. J Mol Struct. 2021;1223:129208. doi:10.1016/j.molstruc.2020.129208
Sharma GD, Pradhan R, Khandelwal K, Singhal R, Liu W, Zhu X, Mishra A. All-small-molecule efficient ternary organic solar cells employing a coumarin donor and two fullerene-free acceptors. J Mater Chem C. 2023;11:1919–1926. doi:10.1039/D2TC05318G
Pradhan R, Khandelwal K, Panda SJ, Purohit CS, Bag BP, Singhal R, Liu W, Zhu X, Sharma GD, Mishra A. Achieving high-efficiency in binary organic solar cells by the structural fine-tuning of coumarin-based donor. Solar RRL. 2023:2300032. doi:10.1002/solr.202300032
Zhao RR, Xu QL, Yang Y, Cao J, Zhou Y, Xu R, Zhang JF. A coumarin-based terpyridine–zinc complex for sensing pyrophosphate and its application in in vivo imaging. Tetrahedron Lett. 2016;57(46):5022–5025. doi:10.1016/j.tetlet.2016.09.081
Feng Z, Yu Y, Yang X, Zhong D, Song D, Yang H, Chen X, Zhou G, Wu Z. Isomers of coumarin-based cyclometalated Ir(III) complexes with easily tuned phosphorescent color and features for highly efficient organic light-emitting diodes. Inorg Chem. 2019;58(11):7393–7408. doi:10.1021/acs.inorgchem.9b00534
Barman S, Mukhopadhyay SK, Gangopadhyay M, Biswas S, Dey S, Singh NDP. Coumarin–benzothiazole–chlorambucil (Cou–Benz–Cbl) conjugate: an ESIPT based pH sensitive photoresponsive drug delivery system. J Mater Chem B. 2015;3(17):3490–3497. doi:10.1039/C4TB02081B
Amin KM, Awadalla FM, Eissa AMA, Abou-Seri SM, Hassan GS. Design, synthesis and vasorelaxant evaluation of novel coumarin–pyrimidine hybrids. Bioorg Med Chem. 2011;19:6087–6097. doi:10.1016/j.bmc.2011.08.037
Chang WR, Lo YH, Lee CY, Wu MJ. Palladium-catalyzed cyclization of enediynes to benzopyranones. Adv Synth Catal. 2008;350:1248–1252. doi:10.1002/adsc.200700516
Charushin V, Chupakhin O. Metal free C–H functionalization of aromatics: nucleophilic displacement of hydrogen. Cham: Springer International Publishing; 2014. 283 p. doi:10.1007/978-3-319-07019-3
Akiba K, Iseki Y, Wada M. Facile synthesis of 4-substituted pyridines using Grignard reagents. Tetrahedron Lett. 1982;23(38):3935–3936. doi:10.1016/S0040-4039(00)87747-3
Fatykhov RF, Sharapov AD, Starnovskaya ES, Shtaitz YK, Savchuk MI, Kopchuk DS, Nikonov IL, Zyryanov GV, Khalymbadzha IA, Chupakhin ON. Coumarin-pyridine push-pull fluorophores: Synthesis and photophysical studies. Spectrochim Acta A. 2022;267:120499. doi:10.1016/j.saa.2021.120499
Utepova IA, Lakhina AE, Varaksin MV, Kovalev IS, Rusinov VL, Slepukhin PA, Kodess MI, Chupakhin ON. Stable σH-adducts in reactions of ferrocenyllithium with azines. Russ Chem Bull. 2009;57(10):2156. doi:10.1007/s11172-008-0292-4
Alphonse FA, Suzenet F, Keromnes A, Lebret B, Guillaumet G. A general approach to selective functionalization of 1,2,4-triazines using organometallics in palladium-catalyzed cross-coupling and addition reactions. Synthesis. 2004(17):2893–2899. doi:10.1055/s-2004-834868
Utepova IA, Musikhina AA, Chupakhin ON, Slepukhin PA. New approach to the synthesis of azinylcymantrenes. Organometallics. 2011;30(11):3047–3053. doi:10.1021/om200159f
Nemytov AI, Utepova IA, Chupakhin ON, Slepukhin PA, Charushin VN. Lithium benzenechromiumtricarbonyl as C-nucleophile in the cross-dehydrogenative coupling reactions of azaaromatics. Inorg Chim Acta. 2019;487:339–344. doi:10.1016/j.ica.2018.12.040
Moseev TD, Varaksin MV, Gorlov DA, Nikiforov EA, Kopchuk DS, Starnovskaya ES, Khasanov AF, Zyryanov GV, Charushin VN, Chupakhin ON. Direct CH/CLi coupling of 1,2,4-triazines with C6F5Li followed by aza-Diels-Alder reaction as a pot, atom, and step economy (PASE) approach towards novel fluorinated 2,2′-bipyridine fluorophores. J Fluor Chem. 2019;224:89–99. doi:10.1016/j.jfluchem.2019.05.008
Berezin MV, Rusinov GL, Charushin VN. C–H-Functionalization of 1,2,4-triazines: oxidation and elimination pathways of aromatization of σH-adducts. Russ Chem Bull. 2014;63(6):1359–1363. doi:10.1007/s11172-014-0603-x
Fatykhov RF, Khalymbadzha IA, Sharapov AD, Potapova AP, Mochulskaya NN, Tsmokalyuk AN, Ivoilova AV, Mozharovskaia PN, Santra S, Chupakhin ON. MnO2-mediated oxidative cyclization of “formal” Schiff’s bases: easy access to diverse naphthofuro-annulated triazines. Molecules. 2022;27(20):7105. doi:10.3390/molecules27207105
Fatykhov RF, Savchuk MI, Starnovskaya ES, Bobkina MI, Kopchuk DS, Nosova EV, Zyryanov GV, Khalymbadzha IA, Chupakhin ON, Charushin VN, Kartsev VG. Nucleophilic substitution of hydrogen–the Boger reaction sequence as an approach towards 8-(pyridin-2-yl)coumarins. Mendeleev Commun. 2019;29(3):299–300. doi:10.1016/j.mencom.2019.05.019
Santana L, González-Díaz H, Quezada E, Uriarte E, Yáñez M, Viña D, Orallo F. Quantitative structure− activity relationship and complex network approach to monoamine oxidase A and B inhibitors. J Med Chem. 2008;51:6740–6751. doi:10.1021/jm800656v
Garazd YL, Garazd MM, Khilya VP. Modified Coumarins. 16. cyclohexane-annelated analogs of pyranocoumarins. Chem Nat Compd. 2005;41:388–395. doi:10.1007/s10600-005-0159-y
Walker D, Hiebert JD. 2,3-Dichloro-5,6-dicyanobenzoquinone and its reactions. Chem Rev. 1967;67(2):153–195. doi:10.1021/cr60246a002
Batista VS, Crabtree RH, Konezny SJ, Lucaa OR, Praetorius JM. Oxidative functionalization of benzylic C–H bonds by DDQ. New J Chem. 2012;36:1141–1144. doi:10.1039/C2NJ40021A
Panja S, Ghosh S, Ghosh K. Pyridine/pyridinium symmetrical bisamides as functional materials: aggregation, selective sensing and drug release. New J Chem. 2018;42(8):6488–6497. doi:10.1039/C7NJ03931J
Kim H, Sarkar S, Nandy M, Ahn KH. Imidazolyl–benzocoumarins as ratiometric fluorescence probes for biologically extreme acidity. Spectrochim Acta A. 2021;248:119088. doi:10.1016/j.saa.2020.119088
Kim D, Xuan QP, Moon H, Jun YW, Ahn KH. Synthesis of benzocoumarins and characterization of their photophysical properties. Asian J Org Chem. 2014;3(10):1089–1096. doi:10.1002/ajoc.201402107
DOI: https://doi.org/10.15826/chimtech.2023.10.2.05
Copyright (c) 2023 Ramil F. Fatykhov, Igor A. Khalymbadzha, Ainur D. Sharapov, Anastasia P. Potapova, Ekaterina S. Starnovskaya, Dmitry S. Kopchuk, Oleg N. Chupakhin

This work is licensed under a Creative Commons Attribution 4.0 International License.
Chimica Techno Acta, 2014–2025
eISSN 2411-1414
Copyright Notice







