Crystallization kinetics of GdYScAlCo high-entropy bulk metallic glass
Abstract
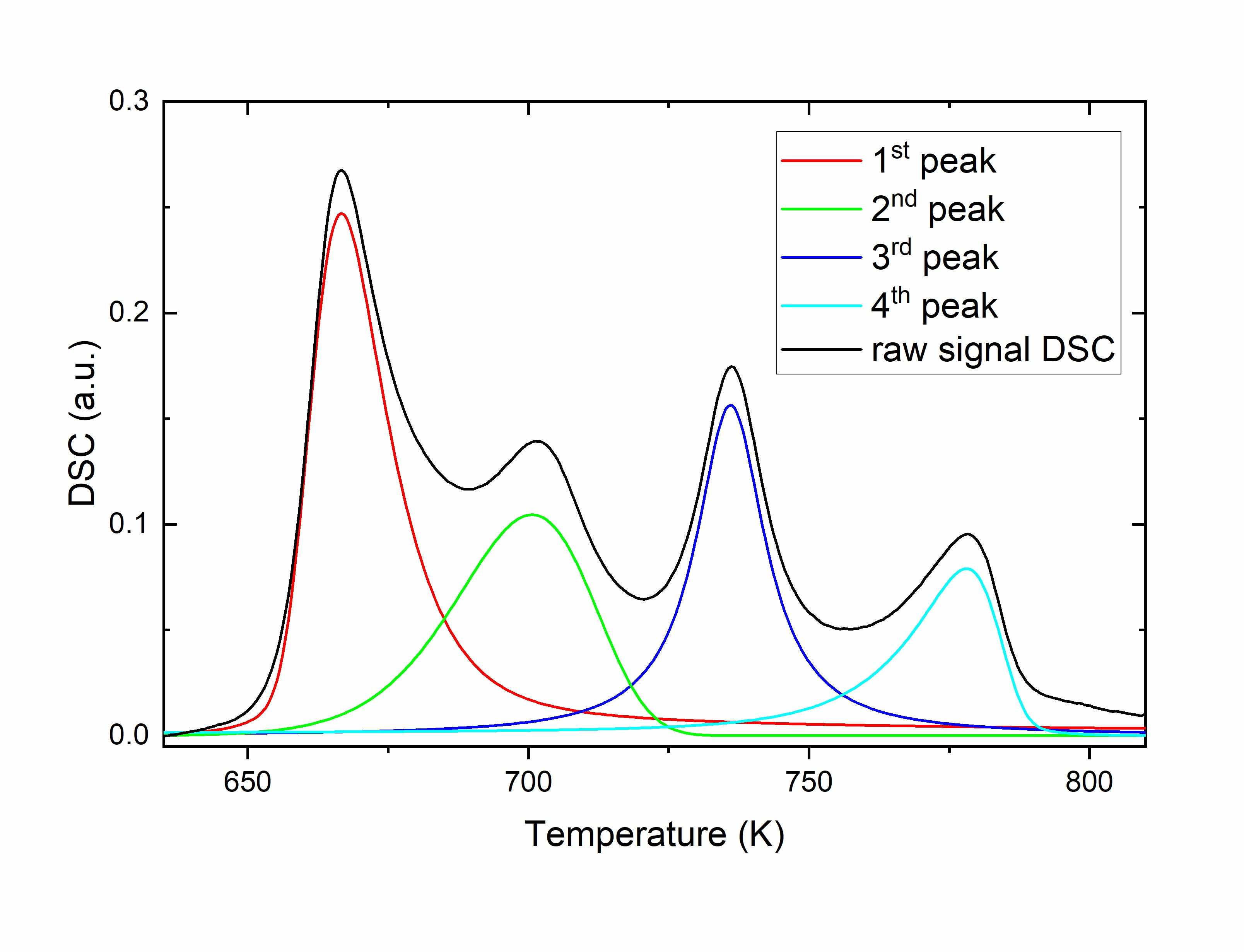
The thermal stability and non-isothermal crystallization of a new bulk-amorphous high-entropy (HE-BMG) equiatomic GdYScAlCo alloy were studied by differential scanning calorimetry (DSC). The alloy shows a four-stage crystallization process. The kinetic parameters (activation energy (Eα)), the pre-exponential factor (logA) and glass-forming ability indicators (kinetic fragility index, characteristic temperatures) for the GdYScAlCo alloy were obtained. The Eα values obtained by isoconversional methods indicate a nonlinear Arrhenian behaviour and a complex process. The Avrami equation modification proposed by Jeziorny and the multivariate nonlinear regression method were applied on the nonisothermal crystallization. In the case of primary crystallization of the amorphous GdYScAlCo alloy under nonisothermal conditions, the kinetics of the nucleation process is best described by an autocatalytic reaction.
Keywords
Full Text:
PDFReferences
Klement W, Willens RH, Duwez Pol. Non-crystalline structure in solidified gold-silicon alloys. Nature. 1960;187:869–870. doi:10.1038/187869b0
Yuan R, Yu Z, Leng H, Chou K. Thermodynamic evaluation and experimental verification of the glass forming ability of Cu–Zr-based alloys. J Non Cryst Solids. 2021;564:120835. doi:10.1016/j.jnoncrysol.2021.120835
Cui X, Zu FQ, Jiang WX, Wang LF, Wang ZZ. Achieving superior glass forming ability of Zr–Cu–Al–Ni–Ti/Ag bulk metallic glasses by element substitution. J Non Cryst Solids. 2013;375:83–87. doi:10.1016/j.jnoncrysol.2013.05.014
Jiang HR, Wei XS, Lu WF, Liang DD, Wen ZH, Xiang HP, Wang Z, Shen J. Design of Cu–Zr–Al and Cu–Zr–Al–Sn bulk amorphous alloys with high glass-forming ability. J Non Cryst Solids. 2019;521:119531. doi:10.1016/j.jnoncrysol.2019.119531
Gao K, Zhu XG, Chen L, Li WH, Xu X, Pan BT, Li WR, Zhou WH, Li L, Huang W, Li Y. Recent development in the application of bulk metallic glasses. J Mater Sci Technol. 2022;131:115–121. doi:10.1016/j.jmst.2022.05.028
Qingjun Chen, Jun Shen, Deliang Zhang, Hongbo Fan, Jianfei Sun, McCartney DG. A new criterion for evaluating the glass-forming ability of bulk metallic glasses. Mater Sci Engin. 2011;21:164–1172. doi:10.1016/j.msea.2006.06.053
Xi XK, Li S, Wang RJ, Zhao DQ, Pan MX, Wang WH. Bulk scandium-based metallic glasses. J Mater Res. 2005;20:2243–2247. doi:10.1557/jmr.2005.0281
Angell CA. Formation of glasses from liquids and biopolymers. Sci. 1995;267:1924–1935. doi:10.1126/science.267.5206.1924
Ruocco G, Sciortino F, Zamponi F, De Michele C, Scopigno T. Landscapes and fragilities. J Chem Phys. 2004;120(22):10666. doi:10.1063/1.1736628
Brüning R, Samwer K. Glass transition on long time scales Phys Rev B. 1992;46(18):11318-11322. doi:10.1103/PhysRevB.46.11318
Senkov ON, Scott JM, Miracle DB. Effect of Al addition of glass forming ability of Ca–Mg–Zn–Cu based bulk metallic glasses. Metall Mater Transact. 2007;39:1901–1907. doi:10.1007/s11661-007-9255-x
NETSCH Peak Separation 3. URL: https://analyzing-testing.netzsch.com/ru/blog/2021/separating-overlapping-effects-in-analytical-measurement-curves
Vyazovkin S. Kissinger method in kinetics of materials: things to beware and be aware of. Molec. 2020;25:2813. doi:10.3390/molecules25122813
Vyazovkin S. Determining Preexponential Factor in Model-Free Kinetic Methods: How and Why? Molec. 2021;26:3077. doi:10.3390/molecules26113077
Vyazovkin S. Evaluation of activation energy of thermally stimulated solid-state reactions under arbitrary variation of temperature. J Comput Chem. 1997;18:393–402. doi:10.1002/(SICI)1096-987X(199702)18:3<393::AID-JCC9>3.0.CO;2-P
Friedman HL. Kinetics of thermal degradation of char-forming plastics from thermogravimetry. J Polym Sci Part C Polym Symposia. 1964;6(1):183–185. doi:10.1002/polc.5070060121
Ozawa Takeo. A new method of analysis thermogravimetric data. bulletin of the chemical society of Japan. Bull Chem Soc Japan. 1965;38:1881–1886. doi:10.1246/bcsj.38.1881
Cheng YT, Hung TH, Huang JC, Hsieh PJ, Jang JSC. Thermal stability and crystallization kinetics of Mg–Cu–Y–B quaternary alloys. Mater Sci Engin. 2007;449:501–505. doi:10.2320/matertrans1989.32.609
Matusita K, Komatsu T, Yokota R. Kinetics of non-isothermal crystallization process and activation energy for crystal growth in amorphous materials. J Mater Sci. 1984;19(1):291–296. doi:10.1007/bf02403137
Avrami M. Kinetics of phase change. I: General theory. J Chem Phys. 1939;7:1103–1112. doi:10.1063/1.1750380
Avrami M. Kinetics of phase change. II Transformation-time relations for random distribution of nuclei. J Chem Phys. 1940;8:212–224. doi:10.1063/1.1750631
Avrami M. Granulation, phase change, and microstructure kinetics of phase change. J Chem Phys. 1941:177–184. doi:10.1063/1.1750872
Shiryayev AN. On the statistical theory of metal crystallization (Ed.), in: A. N. Shiryayev (Ed.), Sel. Work. A. N. Kolmogorov Vol. II Probab. Theory Math Stat. 1992:188–192. doi:10.1007/978-94-011-2260-3_22
Barmak K. A commentary on reaction kinetics in processes of nucleation and growth. Mater Sci. 2010;41:2711–2775. doi:10.1007/s11661-010-0421-1
Erofe’ev BV. Generalized equation of chemical kinetics and its application in reactions involving solids. Dokl Akad Nauk SSSR. 1946:511–514.
Shan Zhang, Chao Wei, Jingwang Lv, Haoran Zhang, Zhilin Sh, Xinyu Zhang, Mingzhen Ma. Non-isothermal crystallization kinetics of the Zr50Cu34Al8Ag8 amorphous alloy. Mater Lett. 2022;307. doi:10.1016/j.matlet.2021.130996
Ruitenberg G, Woldt E, Petford-Long AK. Comparing the Johnson-Mehl-Avrami-Kolmogorov equations for isothermal and linear heating conditions. Thermochim Acta. 2001;378:97–105. doi:10.1016/S0040-6031(01)00584-6
Jeziorny A. Parameters characterizing the kinetics of the non-isothermal crystallization of poly(ethylene terephthalate) determined by d.s.c. Polymer (Guildf). 1978;19:1142–1144. doi:10.1016/0032-3861(78)90060-5
Vyazovkin S. Jeziorny method should be avoided in avrami analysis of nonisothermal crystallization. Polym. 2023;15:197. doi:10.3390/polym15010197
Ozawa T. Kinetics of non-isothermal crystallization. Polymer. 1971;12;3:150–158. doi:10.1016/0032-3861(71)90041-3
Blázquez JS, Conde CF, Conde A. Non-isothermal approach to isokinetic crystallization processes: Application to the nanocrystallization of HITPERM alloys. Acta Mater. 2005;53(8):2305–2311. doi:10.1016/j.actamat.2005.01.037
Opfermann J. Kinetic analysis using multivariate non-linear regression. I. Basic concepts. J Therm Anal Calorim. 2000;60:641–658. doi:10.1023/A:1010167626551
Sourour S, Kamal MR. Differential scanning calorimetry of epoxy cure: isothermal cure kinetics. Thermochim Acta. 1976;14(1–2):41–59. doi:10.1016/0040-6031(76)80056-1
DOI: https://doi.org/10.15826/chimtech.2023.10.2.07
Copyright (c) 2023 V.A. Bykov, D.A. Kovalenko, E.V. Sterkhov, T.V. Kulikova

This work is licensed under a Creative Commons Attribution 4.0 International License.
Chimica Techno Acta, 2014–2025
eISSN 2411-1414
Copyright Notice







